7 How would you expect the molecule 1,10-phenanthroline (shown below) to function as a ligand? H H H H 33847 ㅋ H It would be expected to be a ambidentate ligand. It would be expected to be a bidentate ligand. It would not be expected to function as a ligand. It would be expected to be a monodentate ligand. O
7 How would you expect the molecule 1,10-phenanthroline (shown below) to function as a ligand? H H H H 33847 ㅋ H It would be expected to be a ambidentate ligand. It would be expected to be a bidentate ligand. It would not be expected to function as a ligand. It would be expected to be a monodentate ligand. O
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter20: Chemistry Of Selected Transition Elements And Coordination Compounds
Section: Chapter Questions
Problem 105QRT
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
PLEASE SKIP IF YOU HAVE ALREADY DONE THIS. Thank you
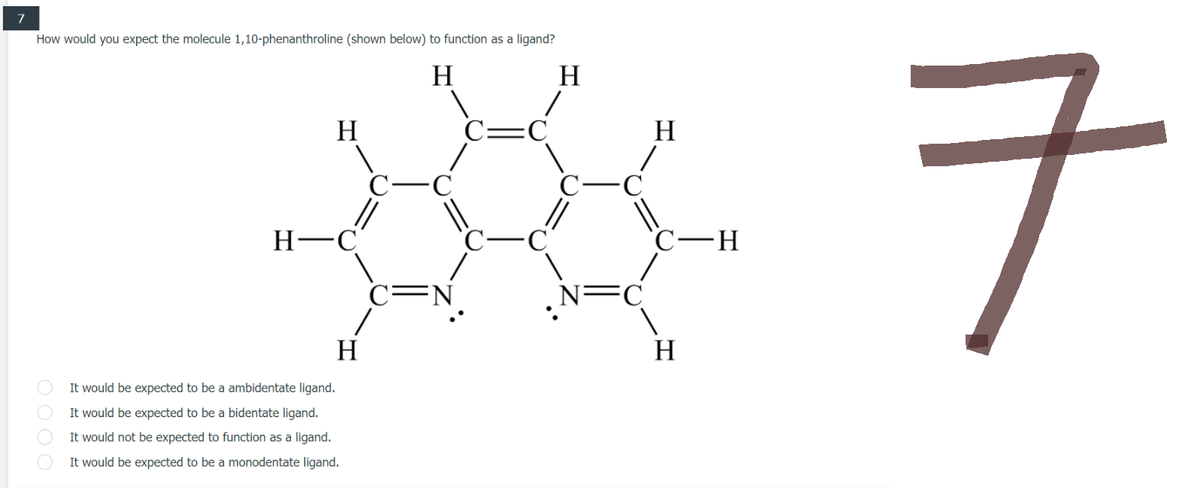
Transcribed Image Text:7
How would you expect the molecule 1,10-phenanthroline (shown below) to function as a ligand?
H
H
H
C=C
H-C
H
It would be expected to be a ambidentate ligand.
It would be expected to be a bidentate ligand.
It would not be expected to function as a ligand.
It would be expected to be a monodentate ligand.
0000
FN
-C
||
H
/
||
FC
C–H
H
ㅋ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning