7. A radioactive form of sodium pertechnetate is used as a brain-scanning agent in medical diagnosis. An analysis of a 0.9872 gram sample found 0.1220 grams of sodium and 0.5255 grams of technetium. The remainder is oxygen. Calculate the empirical formula of sodium pertechnetate. (Use the value of 98.91 as the atomic weight of Tc and arrange the atomic symbols in the formula in the order NaTcO.)
7. A radioactive form of sodium pertechnetate is used as a brain-scanning agent in medical diagnosis. An analysis of a 0.9872 gram sample found 0.1220 grams of sodium and 0.5255 grams of technetium. The remainder is oxygen. Calculate the empirical formula of sodium pertechnetate. (Use the value of 98.91 as the atomic weight of Tc and arrange the atomic symbols in the formula in the order NaTcO.)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 9P: Fulgurites are the products of the melting that occurs when lightning strikes the earth. Microscopic...
Related questions
Question
100%
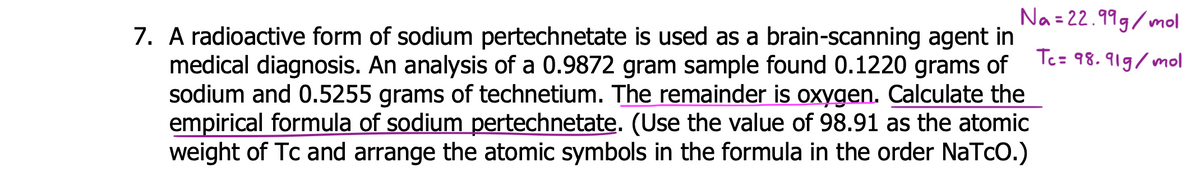
Transcribed Image Text:Na = 22.999/mol
7. A radioactive form of sodium pertechnetate is used as a brain-scanning agent in
medical diagnosis. An analysis of a 0.9872 gram sample found 0.1220 grams of
sodium and 0.5255 grams of technetium. The remainder is oxygen. Calculate the
empirical formula of sodium pertechnetate. (Use the value of 98.91 as the atomic
weight of Tc and arrange the atomic symbols in the formula in the order NaTcO.)
Tc= 98.919/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning