7. Explain why you don't need to specify the number of ions in the compound you are naming ionic substances like those in Model 2. 8. Describe how the names of the nonmetal elements in Model 2 are changed when they are in their anion forms.
7. Explain why you don't need to specify the number of ions in the compound you are naming ionic substances like those in Model 2. 8. Describe how the names of the nonmetal elements in Model 2 are changed when they are in their anion forms.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter5: Nomenclature
Section5.2: Naming Binary Compounds That Contain A Metal And A Nonmetal (types I And Ii)
Problem 1CT
Related questions
Question
Could you answer these questions in 1 to 2 sentences each?
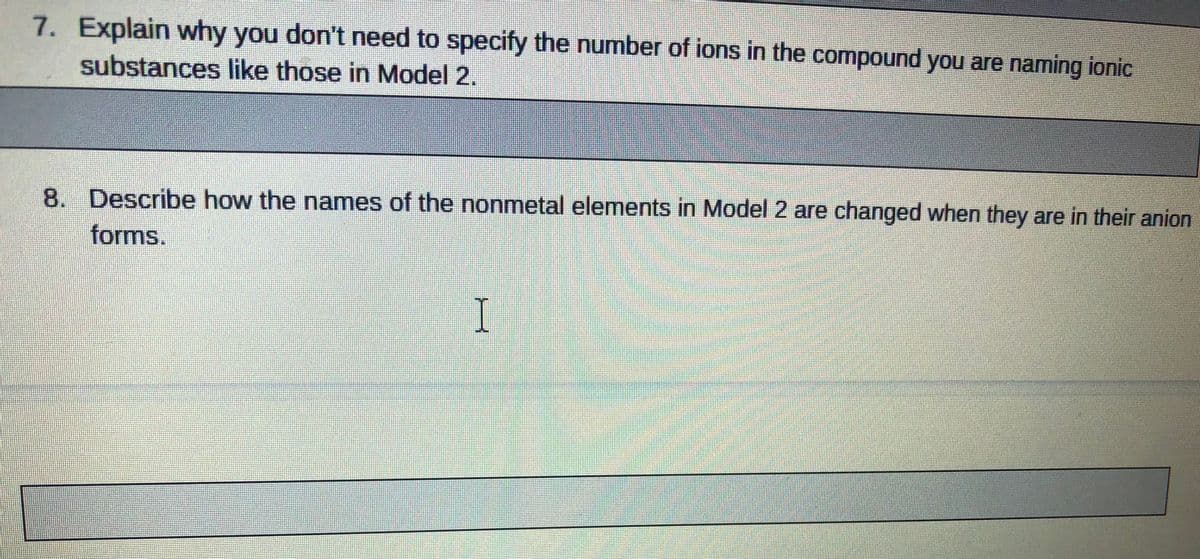
Transcribed Image Text:7. Explain why you don't need to specify the number of ions in the compound you are naming ionic
substances like those in Model 2.
8. Describe how the names of the nonmetal elements in Model 2 are changed when they are in their anion
forms.
I

Transcribed Image Text:Model 2-- lonic Compound Names (Metals that form one ion)
NaCl
Sodium chloride
Zn,P, Zinc phosphide
Al,0, Aluminum oxide
SrCl, Strontium chloride
Cas
Calcium sulfide
Silver sulfide
3.
Ag,S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER