Model 3. Parentheses are used around any ion that is used more than once in a formula unit. Parentheses are used around any polyatomic ion. Parentheses are used around any polyatomic ion used more than once in a formula unit. Parentheses are only used around polyatomic anions used more than once in a formula unit.
Model 3. Parentheses are used around any ion that is used more than once in a formula unit. Parentheses are used around any polyatomic ion. Parentheses are used around any polyatomic ion used more than once in a formula unit. Parentheses are only used around polyatomic anions used more than once in a formula unit.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter5: Resonance
Section: Chapter Questions
Problem 5CTQ
Related questions
Question
100%

Transcribed Image Text:22. Many of the chemical formulas in Model 3 include parentheses. Which one of the following rules
summarizes the appropriate use of parentheses in ternary ionic compounds? For the three rules
that do not apply in all cases, show at least one counter example from the chemical formulas in
Model 3.
Parentheses are used around any ion that is used more than once in a formula unit.
Parentheses are used around any polyatomic ion.
Parentheses are used around any polyatomic ion used more than once in a formula unit.
Parentheses are only used around polyatomic anions used more than once in a formula unit.
STOP
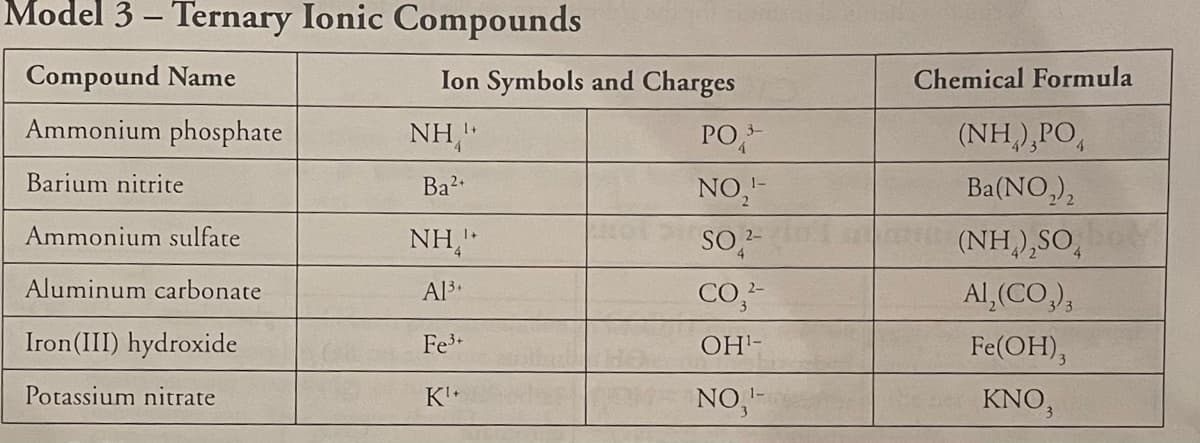
Transcribed Image Text:Model 3 – Ternary Ionic Compounds
Compound Name
Ion Symbols and Charges
Chemical Formula
Ammonium phosphate
NH,"
PO,
(NH,),PO,
Barium nitrite
Ba2
NO,"-
Ba(NO,),
Ammonium sulfate
NH,"
(NH),SO,
Aluminum carbonate
Al3+
CO,-
Al, (CO,),
Iron(III) hydroxide
Fe3
OH-
Fe(OH),
Potassium nitrate
K+
NO,-
KNO,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning