7.When used as electrodes in an electrochemical cell, what two elements would have the greatest electrical voltage? a.aluminum-magnesium or aluminum-zinc, because they would transfer the most electrons per atom b.lithium-gold, because lithium is the strongest reducing agent and gold is the strongest oxidizing agent c.mercury-gold, because they are the two strongest oxidizing agents d.lithium-potassium, because they have the highest chemical activity
7.When used as electrodes in an electrochemical cell, what two elements would have the greatest electrical voltage? a.aluminum-magnesium or aluminum-zinc, because they would transfer the most electrons per atom b.lithium-gold, because lithium is the strongest reducing agent and gold is the strongest oxidizing agent c.mercury-gold, because they are the two strongest oxidizing agents d.lithium-potassium, because they have the highest chemical activity
Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.21QAP
Related questions
Question
7.When used as electrodes in an
a.aluminum-magnesium or aluminum-zinc, because they would transfer the most electrons per atom
b.lithium-gold, because lithium is the strongest reducing agent and gold is the strongest oxidizing agent
c.mercury-gold, because they are the two strongest oxidizing agents
d.lithium-potassium, because they have the highest chemical activity
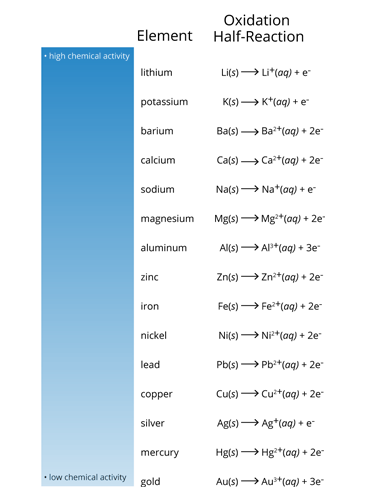
Transcribed Image Text:Oxidation
Half-Reaction
Element
- high chemical activity
lithium
Li(s) » Li*(aq) + e
potassium
K(s) → K*(aq) + e
barium
Ba(s) Ba*(aq) + 2e
calcium
Cals) » Ca*(ag) + 2e
sodium
Na(s) Na*(ag) + e
magnesium
Mg(s) Mg*(aq) + 2e"
aluminum
Al(s) A*(aq) + 3e
zinc
Znls) Zn*(aq) + 2e
iron
Fe(s) Fe*(aq) + 2e
nickel
Ni(s) > Ni*(aq) + 2e
lead
Pb(s) > Pb+(aq) + 2e
copper
Culs) → Cu**(aq) + 2e
silver
Ag(s)→ Ag*(aq) + e
mercury
Hg(s) Hg*(aq) + 2e
• low chemical activity
gold
Au(s) > Aut(aq) + 3e
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning