8) Consider the following graph: n-5 n-4 -52 -82 -146 Brackett series n 3 Paschen series orbital energies kj/mol 328 n-2 Balmer Series -1312 Lyman series a) What is the minimum amount of energy to needed to completely eject an electron of a hydrogen atom from the first energy shell (which is a 1s orbital), to yield a free electron? b) What is the minimum amount of energy needed to promote an electron of an exited hydrogen atom from the 3rd to the 4th energy level? If an electron in an excited hydrogen atom is occupying the 5th energy level and then relaxes back to the ground state, how much energy is released in the form of electromagnetic radiation? c) d) Consider the energy gaps between the energy levels in the graph above. Do the relative energies of the shells converge or diverge as n increases. Why might the observed trend occur? e) Indicate which orbital transitions are associated with the 4 lines in the visible hydrogen line spectrum
8) Consider the following graph: n-5 n-4 -52 -82 -146 Brackett series n 3 Paschen series orbital energies kj/mol 328 n-2 Balmer Series -1312 Lyman series a) What is the minimum amount of energy to needed to completely eject an electron of a hydrogen atom from the first energy shell (which is a 1s orbital), to yield a free electron? b) What is the minimum amount of energy needed to promote an electron of an exited hydrogen atom from the 3rd to the 4th energy level? If an electron in an excited hydrogen atom is occupying the 5th energy level and then relaxes back to the ground state, how much energy is released in the form of electromagnetic radiation? c) d) Consider the energy gaps between the energy levels in the graph above. Do the relative energies of the shells converge or diverge as n increases. Why might the observed trend occur? e) Indicate which orbital transitions are associated with the 4 lines in the visible hydrogen line spectrum
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
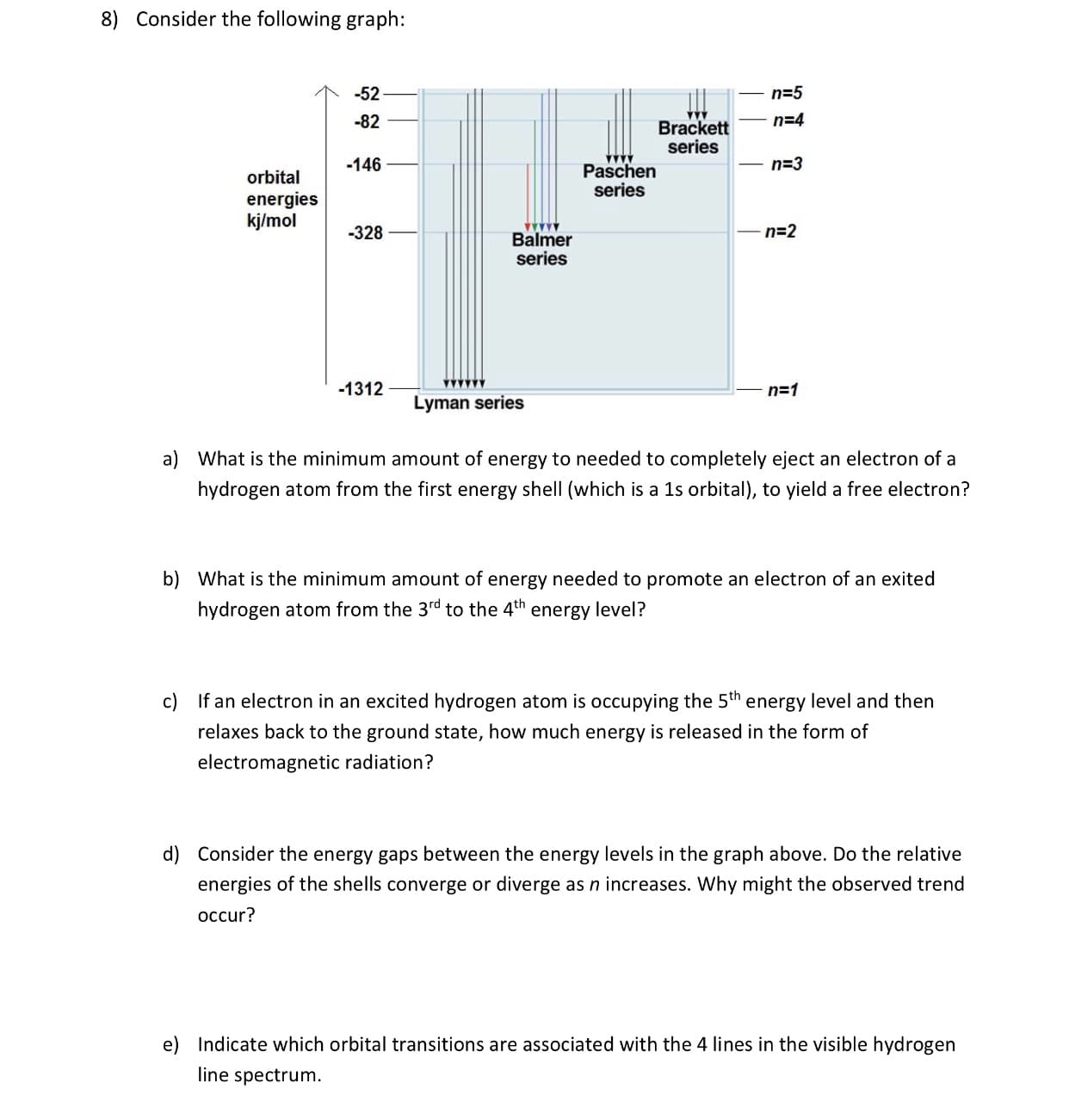
Transcribed Image Text:8)
Consider the following graph:
n-5
n-4
-52
-82
-146
Brackett
series
n 3
Paschen
series
orbital
energies
kj/mol
328
n-2
Balmer
Series
-1312
Lyman series
a)
What is the minimum amount of energy to needed to completely eject an electron of a
hydrogen atom from the first energy shell (which is a 1s orbital), to yield a free electron?
b)
What is the minimum amount of energy needed to promote an electron of an exited
hydrogen atom from the 3rd to the 4th energy level?
If an electron in an excited hydrogen atom is occupying the 5th energy level and then
relaxes back to the ground state, how much energy is released in the form of
electromagnetic radiation?
c)
d)
Consider the energy gaps between the energy levels in the graph above. Do the relative
energies of the shells converge or diverge as n increases. Why might the observed trend
occur?
e)
Indicate which orbital transitions are associated with the 4 lines in the visible hydrogen
line spectrum
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY