8. 2.5 grams of NH4Cl is dissolved in 3 liters of water. The NH4Cl is the solvent b. solute c. solution a. 9. You are given a liquid mixture and told to determine if it is a solution, colloid, or suspension. You notice that no solute particles settle to the bottom as the mixture is left undisturbed. You then shine a laser beam through the mixture and the beam of light is scattered throughout the mixture. The mixture is a b. colloid c. suspension a. solution 10. You are given a solution of NaCl dissolved in water and told to determine if the solution is unsaturated, saturated, or supersaturated. You notice that there is a small layer of NaCl on the bottom of the solution. You then add a few crystals of NaCl and notice that they just sink to the bottom and do not dissolve. The solution is a. unsaturated b. saturated 11. Below is the Lewis structure of ammonia. Would you expect ammonia to dissolve in water? (Hint - First decide if ammonia is polar or nonpolar.) H-N-H H c. supersaturated Yes No
8. 2.5 grams of NH4Cl is dissolved in 3 liters of water. The NH4Cl is the solvent b. solute c. solution a. 9. You are given a liquid mixture and told to determine if it is a solution, colloid, or suspension. You notice that no solute particles settle to the bottom as the mixture is left undisturbed. You then shine a laser beam through the mixture and the beam of light is scattered throughout the mixture. The mixture is a b. colloid c. suspension a. solution 10. You are given a solution of NaCl dissolved in water and told to determine if the solution is unsaturated, saturated, or supersaturated. You notice that there is a small layer of NaCl on the bottom of the solution. You then add a few crystals of NaCl and notice that they just sink to the bottom and do not dissolve. The solution is a. unsaturated b. saturated 11. Below is the Lewis structure of ammonia. Would you expect ammonia to dissolve in water? (Hint - First decide if ammonia is polar or nonpolar.) H-N-H H c. supersaturated Yes No
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 6RQ: In terms of Raoults law, distinguish between an ideal liquid-liquid solution and a nonideal...
Related questions
Question
100%
8-11 please
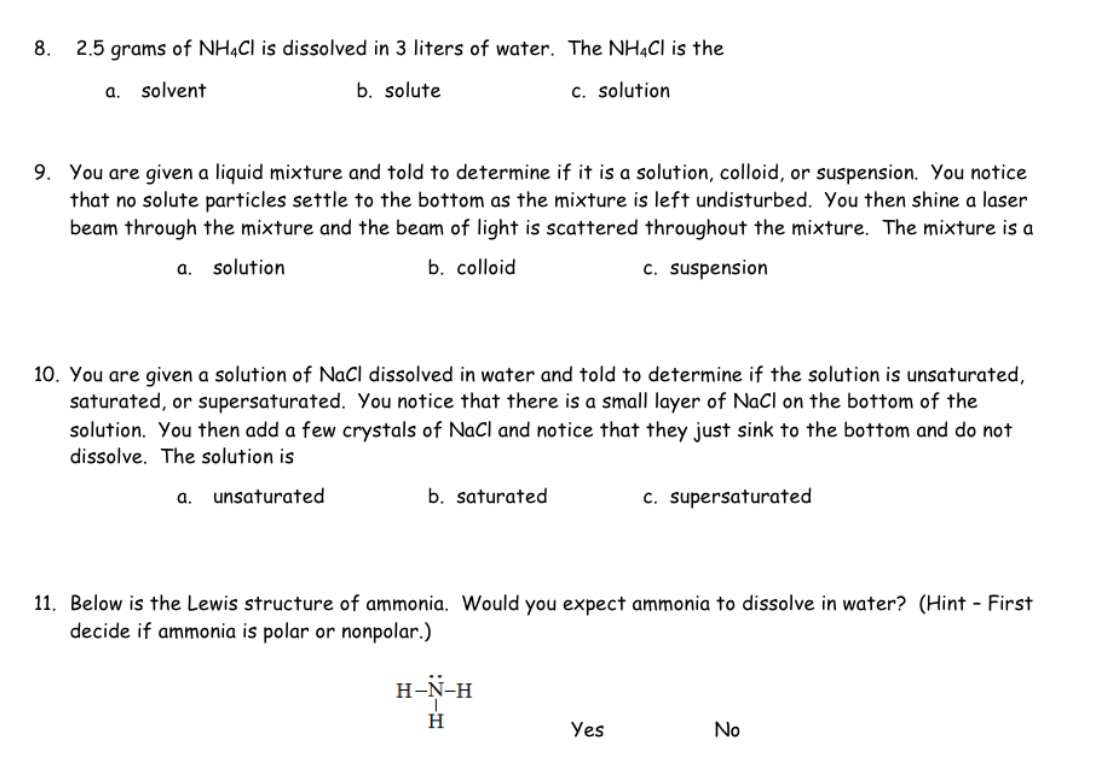
Transcribed Image Text:8. 2.5 grams of NH4Cl is dissolved in 3 liters of water. The NH4Cl is the
solvent
b. solute
c. solution
a.
9. You are given a liquid mixture and told to determine if it is a solution, colloid, or suspension. You notice
that no solute particles settle to the bottom as the mixture is left undisturbed. You then shine a laser
beam through the mixture and the beam of light is scattered throughout the mixture. The mixture is a
b. colloid
c. suspension
a. solution
10. You are given a solution of NaCl dissolved in water and told to determine if the solution is unsaturated,
saturated, or supersaturated. You notice that there is a small layer of NaCl on the bottom of the
solution. You then add a few crystals of NaCl and notice that they just sink to the bottom and do not
dissolve. The solution is
a.
unsaturated
b. saturated
11. Below is the Lewis structure of ammonia. Would you expect ammonia to dissolve in water? (Hint - First
decide if ammonia is polar or nonpolar.)
H-N-H
H
c. supersaturated
Yes
No
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning