Describe how you would (carefully!) prepare 25.00 mL of an aqueous calicheamicin gamma-1 solution that could kill 1.0×10^8 bacteria, starting from a 5.00×10−9M stock solution of the antibiotic. Complete the explanation.
Describe how you would (carefully!) prepare 25.00 mL of an aqueous calicheamicin gamma-1 solution that could kill 1.0×10^8 bacteria, starting from a 5.00×10−9M stock solution of the antibiotic. Complete the explanation.
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.1E
Related questions
Question
Describe how you would (carefully!) prepare 25.00 mL of an aqueous calicheamicin gamma-1 solution that could kill 1.0×10^8 bacteria, starting from a 5.00×10−9M stock solution of the antibiotic. Complete the explanation.
Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. Words may be used more than once.
The 25.00 mL of antibiotic solution needs to contain a minimum of ______
molecules of the drug. Calculate the_____ of drug this represents. The concentration of the stock solution is ______ Then, L stock solution = mol drug/M solution; L = mol/________M.
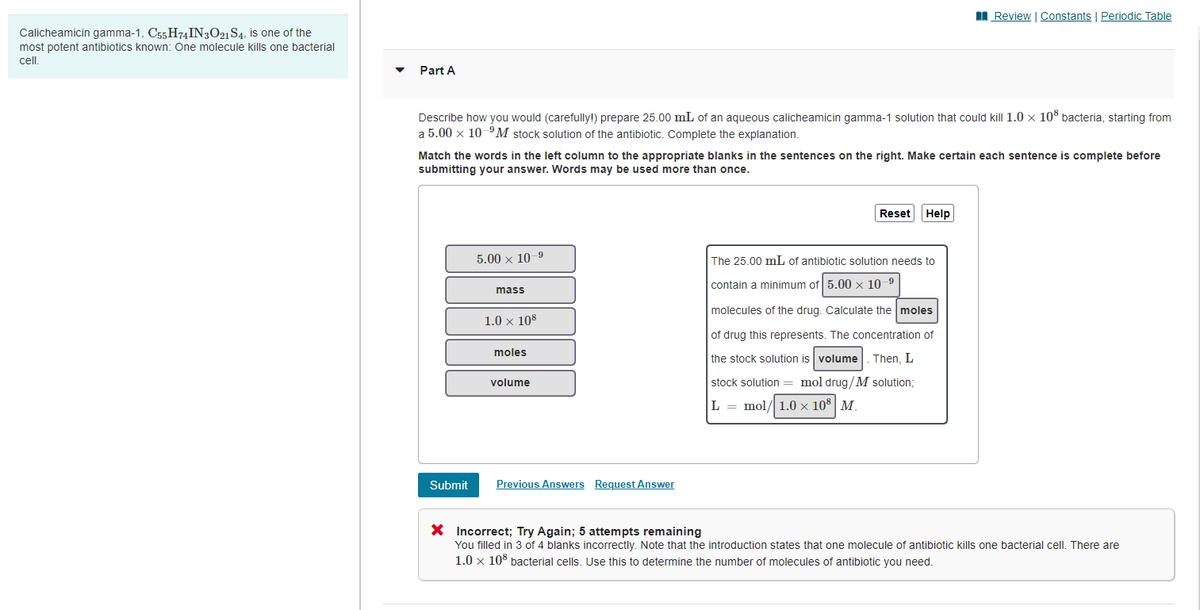
Transcribed Image Text:I Review | Constants | Periodic Table
Calicheamicin gamma-1, C55 H74IN3O21 S4, is one of the
most potent antibiotics known: One molecule kills one bacterial
cel.
Part A
Describe how you would (carefully!) prepare 25.00 mL of an aqueous calicheamicin gamma-1 solution that could kill 1.0 x 10° bacteria, starting from
a 5.00 x 10 "M stock solution of the antibiotic. Complete the explanation.
Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before
submitting your answer. Words may be used more than once.
Reset
Help
5.00 x 10-9
The 25.00 mL of antibiotic solution needs to
contain a minimum of 5.00 x 10 9
mass
molecules of the drug. Calculate the moles
1.0 x 108
of drug this represents. The concentration of
moles
the stock solution is volume
Then, L
volume
stock solution = mol drug/M solution;
L = mol/ 1.0 × 10 | M.
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
You filled in 3 of 4 blanks incorrectly. Note that the introduction states that one molecule of antibiotic kills one bacterial cell. There are
1.0 x 10% bacterial cells. Use this to determine the number of molecules of antibiotic you need.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning