8. Given the following information: Li(s) HI(g) → H(g) + I(g) enthalpy of sublimation of Li(s) = 166 kJ/mol bond energy of HI = 295 kJ/mol Li(g) Li(g) → Li"(g) + e ionization energy of Li(g)= 520. kJ/mol I(g) + e — Г(g) electron affinity of I(g) = -295 kJ/mol Li"(g) + I(g) → LiI(s) lattice energy of LiI(s) = -737 kJ/mol H2(g) → 2H(g) Calculate the change in enthalpy for: bond energy of H2 = 432 kJ/mol 2Li(s) + 2HI(g) –→ H2(g) + 2LİI(s) a. 330 kJ b. –534 kJ c. -483 kJ d. -984 kJ e. none of these
8. Given the following information: Li(s) HI(g) → H(g) + I(g) enthalpy of sublimation of Li(s) = 166 kJ/mol bond energy of HI = 295 kJ/mol Li(g) Li(g) → Li"(g) + e ionization energy of Li(g)= 520. kJ/mol I(g) + e — Г(g) electron affinity of I(g) = -295 kJ/mol Li"(g) + I(g) → LiI(s) lattice energy of LiI(s) = -737 kJ/mol H2(g) → 2H(g) Calculate the change in enthalpy for: bond energy of H2 = 432 kJ/mol 2Li(s) + 2HI(g) –→ H2(g) + 2LİI(s) a. 330 kJ b. –534 kJ c. -483 kJ d. -984 kJ e. none of these
Chapter7: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 126E: Using data from the text, determine the following values (justify your answer): a. the electron...
Related questions
Question
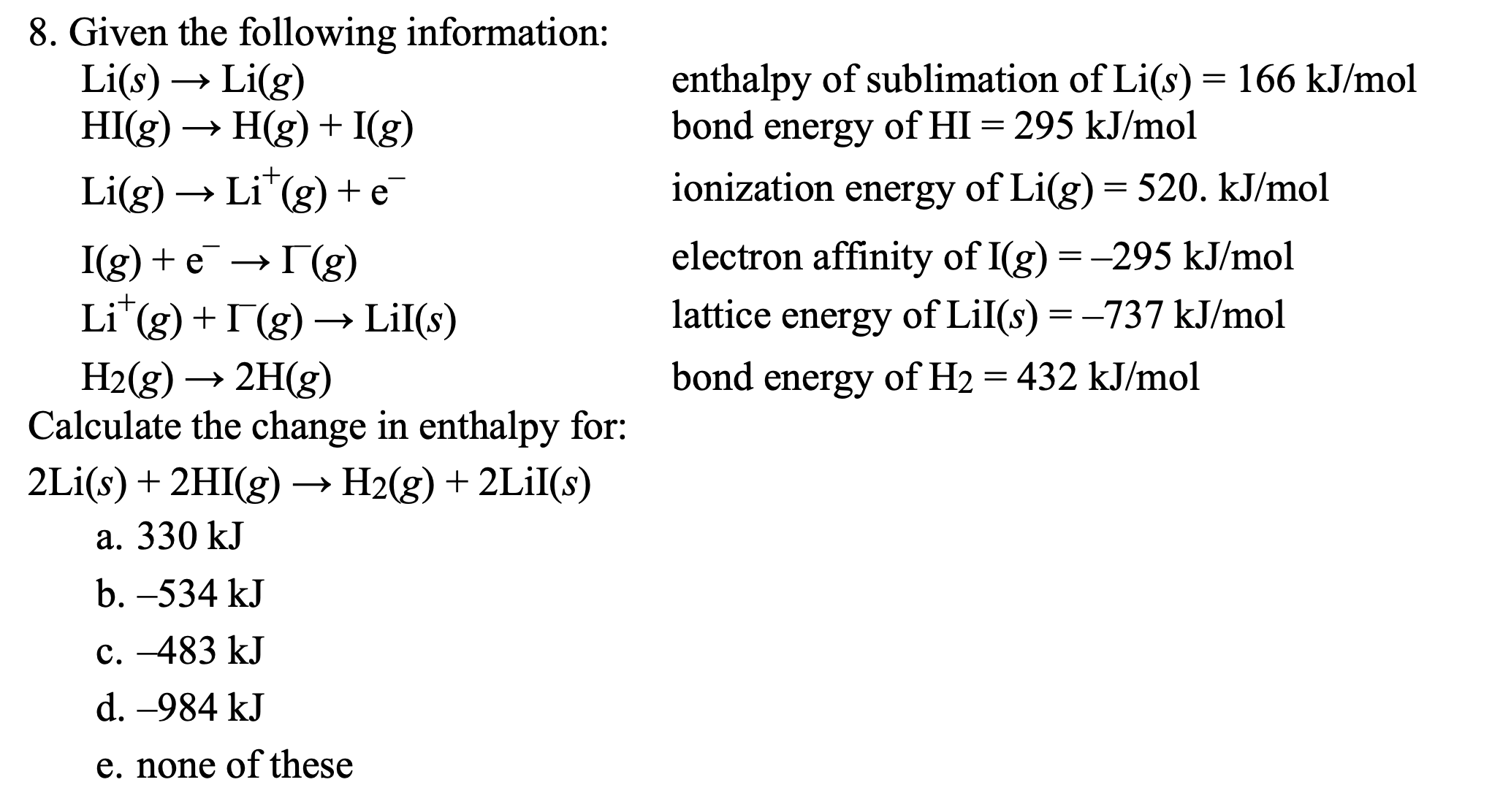
Transcribed Image Text:8. Given the following information:
Li(s)
HI(g) → H(g) + I(g)
enthalpy of sublimation of Li(s) = 166 kJ/mol
bond energy of HI = 295 kJ/mol
Li(g)
Li(g) → Li"(g) + e
ionization
energy of Li(g)= 520. kJ/mol
I(g) + e — Г(g)
electron affinity of I(g) = -295 kJ/mol
Li"(g) + I(g) → LiI(s)
lattice energy of LiI(s) = -737 kJ/mol
H2(g) → 2H(g)
Calculate the change in enthalpy for:
bond energy of H2 = 432 kJ/mol
2Li(s) + 2HI(g) –→ H2(g) + 2LİI(s)
a. 330 kJ
b. –534 kJ
c. -483 kJ
d. -984 kJ
e. none of these
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 6 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning