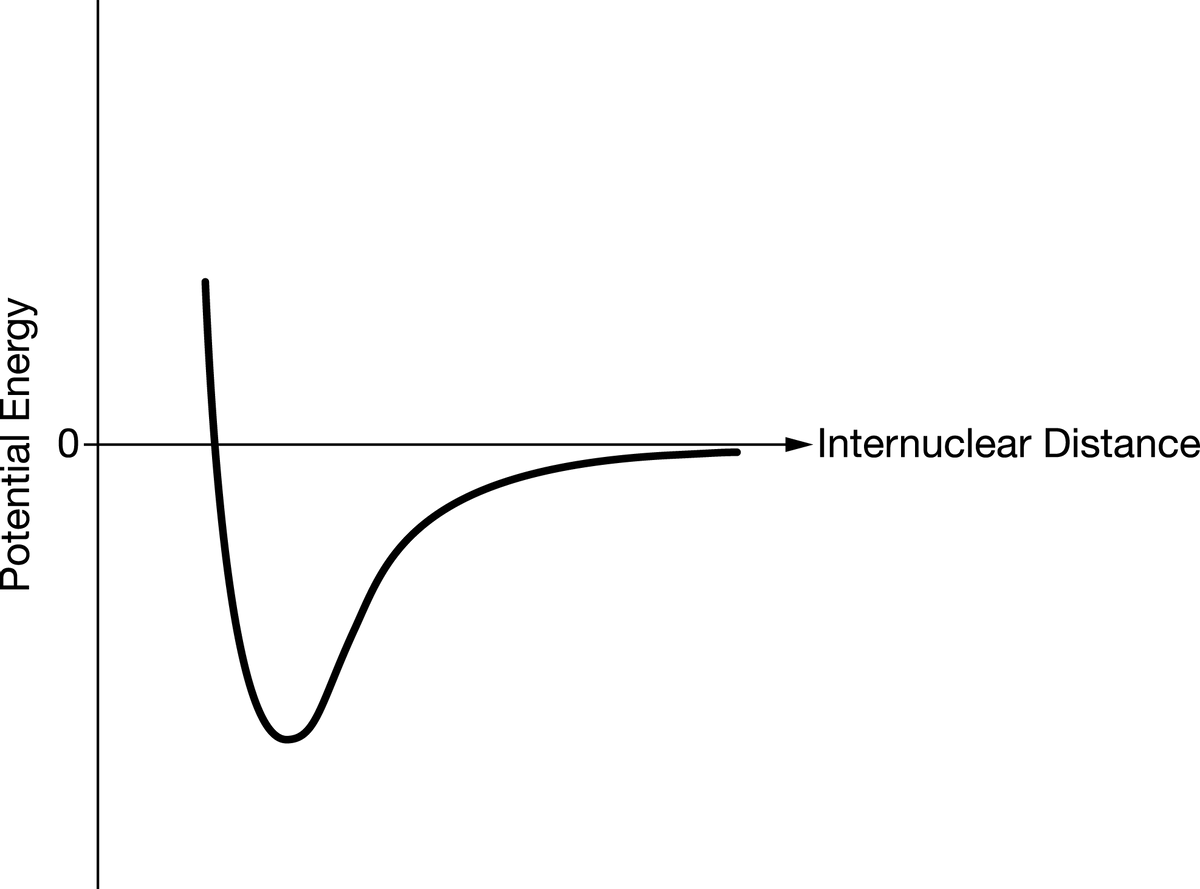
Answer the following questions related to the chemical bonding in substances containing Cl. (a) What type of chemical bond is present in the Cl2 molecule? (b) Cl2 reacts with the element Sr to form an ionic compound. Based on periodic properties, identify a molecule, X2, that is likely to react with Sr in a way similar to how Cl2 reacts with Sr. Justify your choice. (c) A graph of potential energy versus internuclear distance for two Cl atoms is given below. On the same graph, carefully sketch a curve that corresponds to potential energy versus internuclear distance for two Br atoms.
Item 5
Answer the following questions related to the
(a) What type of chemical bond is present in the Cl2 molecule?
(b) Cl2 reacts with the element Sr to form an ionic compound. Based on periodic properties, identify a molecule, X2, that is likely to react with Sr in a way similar to how Cl2 reacts with Sr. Justify your choice.
(c) A graph of potential energy versus internuclear distance for two Cl atoms is given below. On the same graph, carefully sketch a curve that corresponds to potential energy versus internuclear distance for two Br atoms.
(e) Answer the following based on the diagram you drew above.
(i) What is the hybridization of the CC atoms in C2Cl4?
(ii) What is the approximate chlorine-carbon-chlorine bond angle in C2Cl4?
(iii) Is the C2Cl4 molecule polar?
Image for part c.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images






