Consider the following data ior yttrium: 88.906 mol atomic mass electronegativity 1.22 kJ 29.6 mol electron affinity kJ 600. mol ionization energy kJ 11.4 mol heat of fusion
Consider the following data ior yttrium: 88.906 mol atomic mass electronegativity 1.22 kJ 29.6 mol electron affinity kJ 600. mol ionization energy kJ 11.4 mol heat of fusion
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 78E
Related questions
Question
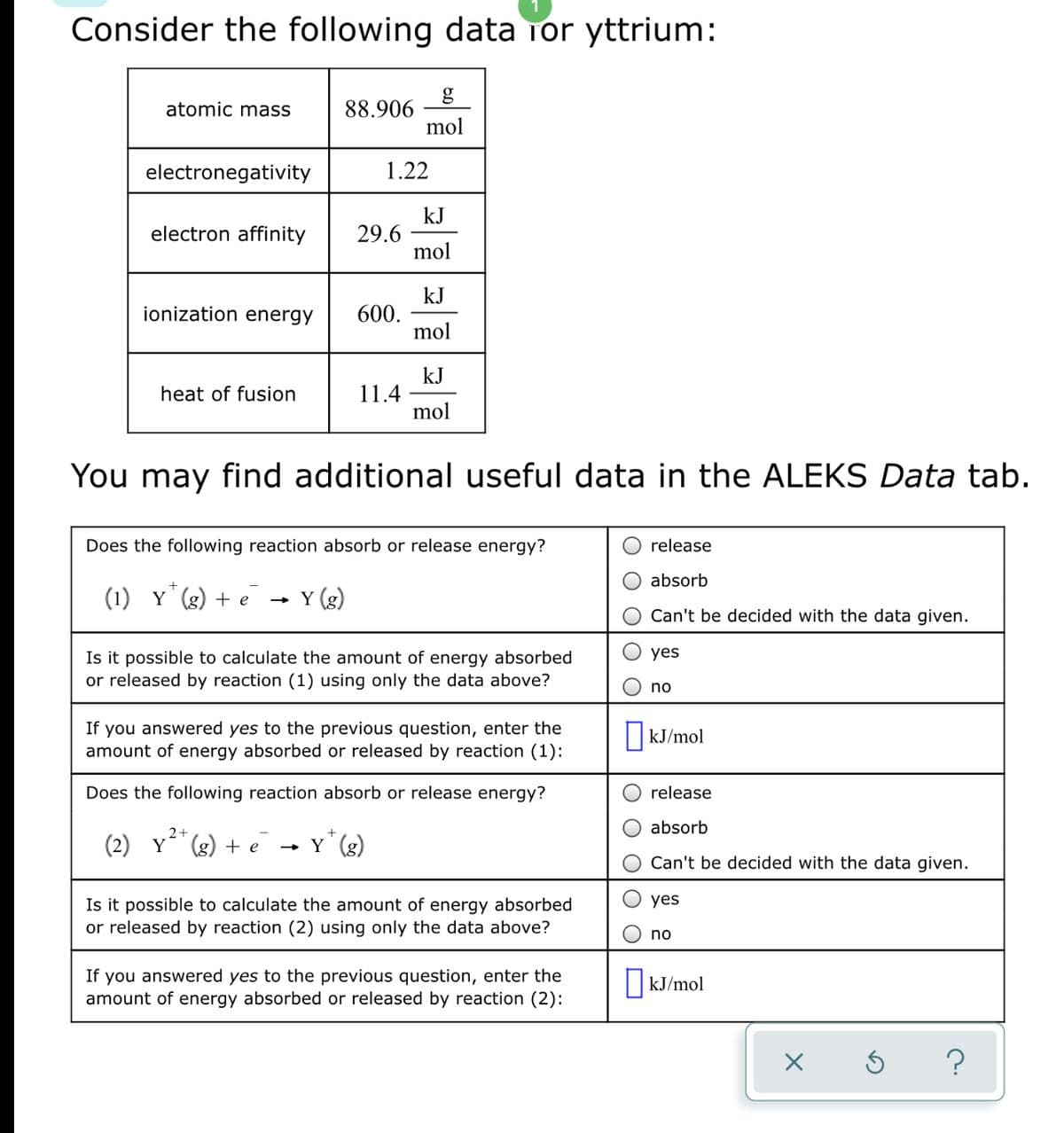
Transcribed Image Text:Consider the following data Tor yttrium:
88.906
mol
atomic mass
electronegativity
1.22
kJ
29.6
mol
electron affinity
kJ
600.
mol
ionization energy
kJ
11.4
mol
heat of fusion
You may find additional useful data in the ALEKS Data tab.
Does the following reaction absorb or release energy?
release
absorb
(1) Y (g) + e
Y (g)
Can't be decided with the data given.
yes
Is it possible to calculate the amount of energy absorbed
or released by reaction (1) using only the data above?
no
If you answered yes to the previous question, enter the
amount of energy absorbed or released by reaction (1):
kJ/mol
Does the following reaction absorb or release energy?
release
absorb
2+
(2) Y (g) + e
- Y (g)
Can't be decided with the data given.
yes
Is it possible to calculate the amount of energy absorbed
or released by reaction (2) using only the data above?
no
If you answered yes to the previous question, enter the
amount of energy absorbed or released by reaction (2):
|KJ/mol
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning