800 A в 600 400 200 20 40 60 80 100 Temperature (°C) Choose the best answer from the drop down menu. Using the graph above, what chemical has the weakest intermolecular force? Vapor pressure (torr)
800 A в 600 400 200 20 40 60 80 100 Temperature (°C) Choose the best answer from the drop down menu. Using the graph above, what chemical has the weakest intermolecular force? Vapor pressure (torr)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.34PAE: 9.34 A copper nail and an iron nail of the same mass and initially at the same room temperature are...
Related questions
Question
Please answer both of these question, each are on different attachments. Please show all work and steps. Thank you!!
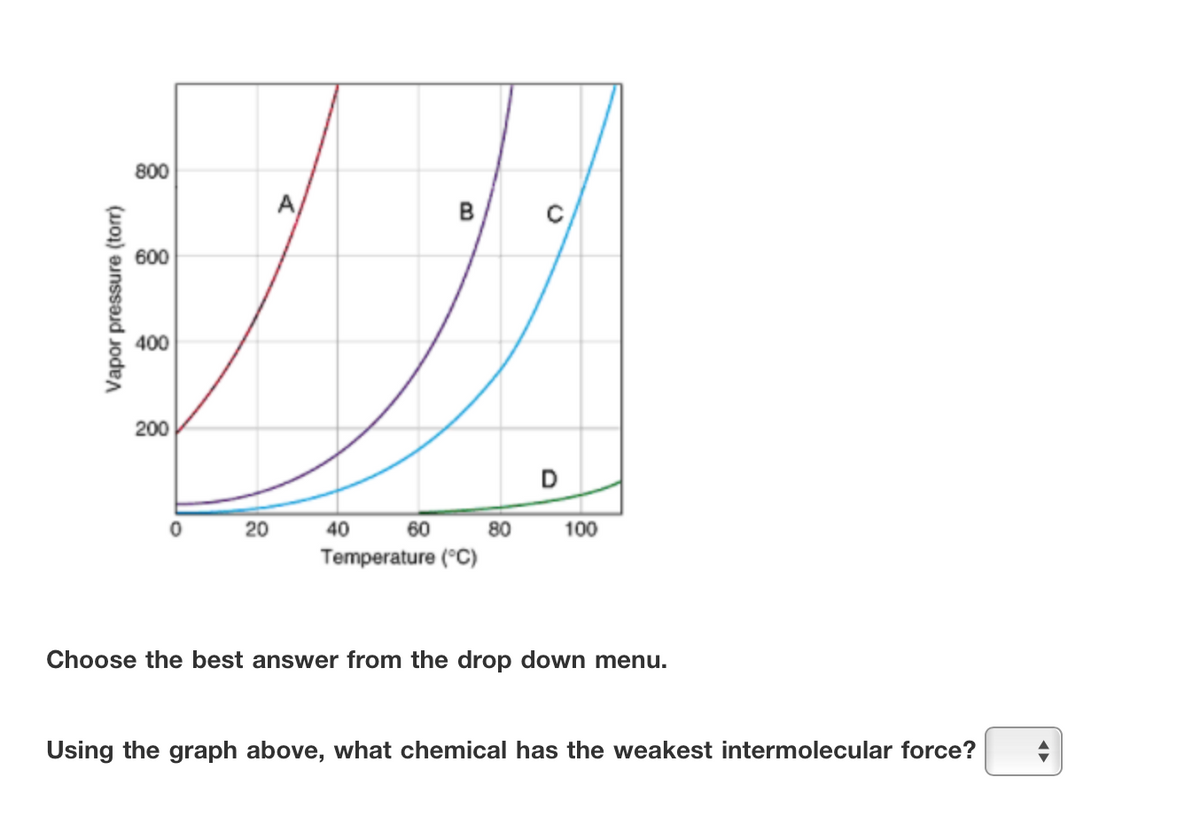
Transcribed Image Text:800
A
B
600
400
200
D
20
40
60
80
100
Temperature (°C)
Choose the best answer from the drop down menu.
Using the graph above, what chemical has the weakest intermolecular force?
Vapor pressure (torr)
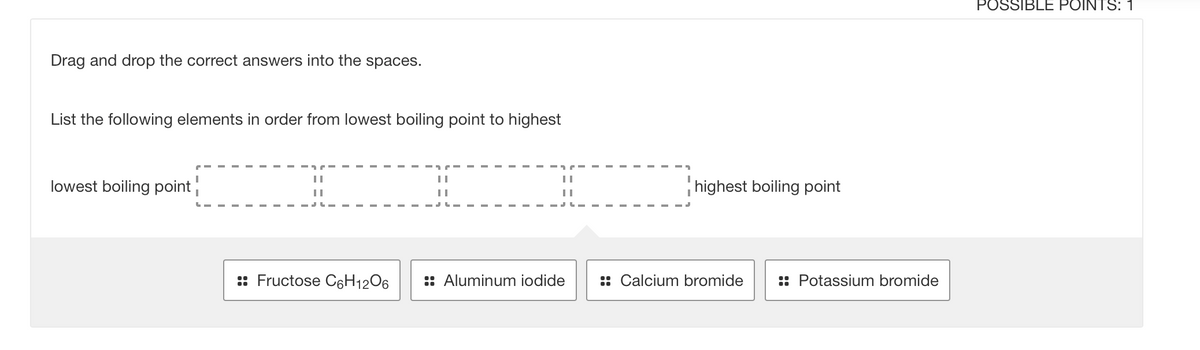
Transcribed Image Text:POSSIBLE POINTS: 1
Drag and drop the correct answers into the spaces.
List the following elements in order from lowest boiling point to highest
lowest boiling point
highest boiling point
:: Fructose C6H12O6
:: Aluminum iodide
:: Calcium bromide
:: Potassium bromide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning