9) Calculate the of the reaction graphite to diamond. trs . At 298K and 100kPa, diamond and graphite's molar entropy and molar combustion heat are listed below. S„ /(J -K"mol*) 2.45 A,H„ (kJ -mol") Diamond -395.40 Graphite 5.71 -393.51
9) Calculate the of the reaction graphite to diamond. trs . At 298K and 100kPa, diamond and graphite's molar entropy and molar combustion heat are listed below. S„ /(J -K"mol*) 2.45 A,H„ (kJ -mol") Diamond -395.40 Graphite 5.71 -393.51
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter8: Bonding: General Concepts
Section: Chapter Questions
Problem 157CP: Calculate the standard heat of formation of the compound ICl(g) at 25C. (Hint: Use Table 8.5 and...
Related questions
Question
100%

Transcribed Image Text:9) Calculate the
of the reaction graphite to diamond.
trs
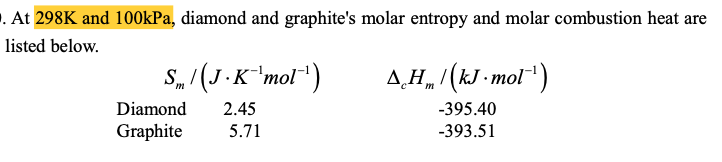
Transcribed Image Text:. At 298K and 100kPa, diamond and graphite's molar entropy and molar combustion heat are
listed below.
S„ /(J -K"mol*)
2.45
A,H„ (kJ -mol")
Diamond
-395.40
Graphite
5.71
-393.51
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning