The standard molar entropy of NH3 is 192.45 J K1 mol at 298K, and its heat capacity is given by the equation Coma +bT +c/T2 with the coefficients given in the table below. Calculate the standard molar entropy of NH3 at 100 °C p,m Please explain as much as possible. Why did you use the equation? Or what conditions did you see from the question? etc. Table 1: Temperature variation of molar heat capacities, Cp,m/U K-1mol-1) a + bT + c/T2 b/(10-3 K-) 25.1 c/(10 K2) a NH3 29.75 -155
The standard molar entropy of NH3 is 192.45 J K1 mol at 298K, and its heat capacity is given by the equation Coma +bT +c/T2 with the coefficients given in the table below. Calculate the standard molar entropy of NH3 at 100 °C p,m Please explain as much as possible. Why did you use the equation? Or what conditions did you see from the question? etc. Table 1: Temperature variation of molar heat capacities, Cp,m/U K-1mol-1) a + bT + c/T2 b/(10-3 K-) 25.1 c/(10 K2) a NH3 29.75 -155
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter16: Thermodynamics: Directionality Of Chemical Reactions
Section: Chapter Questions
Problem 88QRT: Billions of pounds of acetic acid are made each year, much of it by the reaction of methanol with...
Related questions
Question
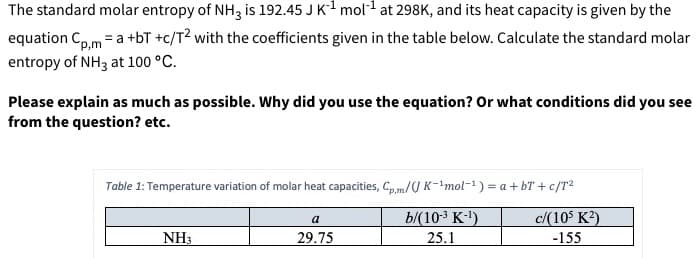
Transcribed Image Text:The standard molar entropy of NH3 is 192.45 J K1 mol at 298K, and its heat capacity is given by the
equation Coma +bT +c/T2 with the coefficients given in the table below. Calculate the standard molar
entropy of NH3 at 100 °C
p,m
Please explain as much as possible. Why did you use the equation? Or what conditions did you see
from the question? etc.
Table 1: Temperature variation of molar heat capacities, Cp,m/U K-1mol-1) a + bT + c/T2
b/(10-3 K-)
25.1
c/(10 K2)
a
NH3
29.75
-155
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning