9. Given the activity series K > Na > Ca > Mg > Al > Sn > Cu > Ag, which of the following metals will reduce Cu2+? a. Sn b. Mg c. K e. all of the above d. Na
Q: Consider the following redox reaction: Fe (s) + Cl2 (g) → FeCl2 (aq) Which of the following…
A: Oxidation :- The process of lose of electrons or increase in oxidation number is called oxidation…
Q: Determine if HNO3 can dissolve metal sample. If it can, writea balanced chemical reaction showing…
A:
Q: Which of the following reaction is NOT going to happen according to non-metal activity series? a 2…
A: Nonmetals are elements that usually react by gaining electrons, rather than by losing electrons…
Q: Use the method of half-reactions to balance the following redox equation in basic solution: Al(s)…
A:
Q: For a particular redox reaction, Cr is oxidized to CrO and Cu²+ is reduced to Cu*. Complete and…
A: Write the balanced redox reaction under basic condition---
Q: Which metals dissolve in HCl? For those metals that do dissolve, write a balanced redox reaction…
A: The given metals: (a) Ag (b) Fe (c) Cu (d) Al To find: The metal that dissolves in HCl and balanced…
Q: + 1.14 V TI Pt Cr3+ (1 M) Cr2+ (1 M) T13+ (1 M) T13+ (aq) + 3e- → TI (s) Cr2+ (aq) → Cr3+ (aq) + e-…
A: An electrochemical cell possesses cathode and anode. A cathode is a metal electrode which is…
Q: In a particular redox reaction, Cr is oxidized to CrO and Cut is reduced to Cu*. Complete and…
A: Given that, for the given redox reaction, 'Cr' is oxidized to CrO42- and Cu2+ is reduced to Cu+…
Q: 1. Is rusting of iron a physical change or chemical change? Explain why? 2. What is the colour of…
A: Rusting : Rusting is a reaction in which iron reacts with water and oxygen to give hydrated iron…
Q: Which of the following metals is most likely to become oxidized (act as O Ca2+(aq)+2e¯→ Ca(s) E°red=…
A: Introduction : Oxidation means loss of electron .
Q: Which one of the following, when added to a piece of Cu(s) in solution, is capable of oxidizing…
A: The reactivity series is of metal is: The metals at the top of the series are powerful reducing…
Q: What is the oxidation number of Zn in zinc metal? (Sec. 17.1) (a) 0 (b) -1 (c) -2 (d) -3 (e) none of…
A: We know that most of the metal showing the multiple oxidation number. Oxidation number is represent…
Q: Among these metals, which is considered soft? a. Cu b. Sn c. Au d. Al Metals are good…
A: A metal can donate electrons from its outermost shell.
Q: Find the reduction and Oxidation. Ag + CN + 02 Ag (CN),
A:
Q: Determine whether or not each metal dissolves in 1 M HNO3. For those metals that do dissolve, write…
A:
Q: Compensate for the following redox reactions using the half reaction method? In Acidic Solution;…
A: (Fe(CN)6)3-+ N2H4→(Fe(CN)6)4-+ N2 Reactant product oxidation number Fe=+3,C=+2 N=-3…
Q: Use the table on the information page to predict if a reaction will occur when Ni(s) and…
A: From the given reactivity table, Ni is above from H that means Ni is more reactive than H. The…
Q: How many electrons are gained or lost by each copper atom In the balanced redox reaction: 2 Cu(s) +…
A: Sulphur is an electron deficient atom (like oxygen). Sulphur has 6 electrons in its valence shell…
Q: For a particular redox reaction, BrO¯ is oxidized to BrO5 and Fe+ is reduced to Fe²+. Complete and…
A: Steps involved: Split reaction into oxidation and reduction halves Balance the oxidation and…
Q: 5. Consider the redox reaction shown below. Which species is reduced? Fe3* (ea + 3Li(s) 3Li* (eg) +…
A: Since in reduction, the specie gains electron from the reaction. hence the specie which is gaining…
Q: Complete and balance the following half-reactions. In each case indicate whether the half-reaction…
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: Balance the following in the type of solution indicated.(a) H2 + Cu2+ ⟶ Cu (acidic solution)(b) H2 +…
A: We can say redox reactions are those chemical reactions which involves transfer of electrons takes…
Q: For a particular redox reaction, NO₂ is oxidized to NO3 and Fe³+ is reduced to Fe²+. Complete and…
A: Oxidation is loss of electrons. Reduction is gain of electrons
Q: Which species in each pair is a better oxidizing agent under standard-state conditions? a) Brz or…
A: The species that has higher value of standard reduction potential will undergo reduction of itself…
Q: Which of the following metals will act spontaneously as reducing agents with silver iodide but not…
A: Given, Options are :
Q: 6. Which metal is the best reducing agent? (a) Ag (b) Mg (c) Fe (d) Pb
A:
Q: Balance the ff. redox reactions. Balance them using water in acidic medium if necessary. Cu+(aq)…
A: The steps involved while balancing a chemical equation by oxidation number method in acid…
Q: Which pair of half-reactions could be used to electroplate silver onto iron? A) reduction: Ag+…
A: According to Activity Series of Metals (in Order of Reactivity) in the Oxidation Half Reaction of…
Q: 0000 Which metal (Cu, Fe, or Al) will reduce water at pH= 0 but will NOT reduce water at pH = 14? 2…
A: Here we have to tell that which metal will reduce water at PH =0 but will not reduce water at PH =…
Q: A sample of 1.55g of iron ore is dissolved in a acid solution in which the iron is converted into…
A: Given : The titration reaction involves oxidation of Fe2+ to Fe3+ using MnO4- ions which is reduced…
Q: Balance the following Redox Reaction. Always include the state of each species: (s), (l), (g), or…
A:
Q: Consider the precipitation reactions below: Fe2* + S? > Fes Cu2+ + S2- →Cus Zn2* + S2 → ZnS…
A:
Q: . Complete and balance the following half-reactions. In each case, indicate whether the…
A:
Q: Determine whether or not each metal dissolves in 1 M HNO3. Forthose metals that dissolve, write a…
A:
Q: Which metal does not dissolve in HCI? Select one: a. Ni Ob.Ag c. Sn d. Mn
A: The given Metals react with HCl following way Ni(s) + 2 HCl(aq) -----> NiCl2(aq) + H2(g) Sn(s) +…
Q: Which of the following metals can be oxidized by H+ under standard conditions? Cu, Zn, Ag, Sn…
A:
Q: Balance the following reaction in acidic solution. Fe?+ (ag) + MnO, (ag) → Fe+ (aq) + Mn²+(aq) Fill…
A: The chemical reaction in which a substance loses an electron is known as oxidation reaction. The…
Q: Half-reaction E° (V) 1(s) + 2e 21'(aq) 0.535V Cu*(aq) + 2e → Cu(s) 0.337V Fe (aq) + 2e Fe(s) -0.440V…
A:
Q: Use the half reaction method to balance the reaction under basic conditions. Sb(s) + Oz(g) → SbO,…
A: From the above given reaction Each step of balancing is shown under half reaction method and…
Q: Irn/takeAssignment/take Lv2 | Online teaching and X Activity Series of Metals…
A: Here, we have to find whether a reaction will occur or not when Ag(s) and hydrobromic acid are…
Q: What is the total number of moles ( n) of electrons exchanged between the oxidizing agent and the…
A: Applying concept of oxidation and reduction.
Q: Balance the following redox reaction in an alkaline solution by the half-reaction method. The final…
A: The answer is given below
Q: Write two half-equations for the following sreactions. For each half-equation state whether…
A:
Q: An external water pipe is built of aluminum (Al). In order to prevent corrosion, the nails that hold…
A: In order to prevent corrosion of aluminium metal pipe, the nails must be made of metal with more…
Q: Why does aluminum (Al) metal not undergo corrosion like iron does? A) Al does not easily react…
A: Aluminium does not undergoes rusting as iron because:
Q: 3. Consider the following galvanic cell: Fe(s) Cr(s) Salt bridge containing KNO,(aq) 1M Fe+ IM Cr+…
A: Given galvanic cell reaction between Fe and Cr. Which are the cathode and anode?
Q: 1.a. 21.5 g of Ag was plated from a solution of AGNO3 with a current of 3.45 amps. a. The moles of…
A: Solution 1a) a) .
Q: 11. What are the factors on which corrosion depends? b. Name three processes used to prevent…
A: Factors on which corrosion depends includes, Exposure to moisture Increase in temperature pH of the…
Q: In a particular redox reaction, Cr is oxidized to CrO and Fe+ is reduced to Fe2+. Complete and…
A:
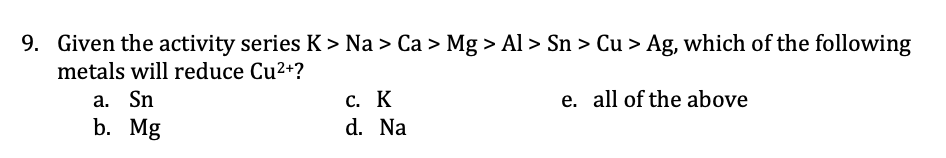
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- Use data from CRC_Std_Thermodyn_Substances and CRC_Std_Thermodyn_Aqueous-Ions to calculate ΔrH∘ΔrH∘ (in kj/mol) for the following at 25 ∘∘C. 2CaC2O4(s)⟶4CO(g)+O2(g)+2CaO(s)a. Give the molar concentration of hydrochloric acid with a specific gravity of 1.18 andhas 37% (w/w) purity. MW=36.5 b. How would you prepare 1.2 L of 0.40 M hydrochloric acid starting from a 2.0 M solution?Preparation of cpper(1) chloride What color observed for the solution of Na2SO3 in water? What colour observed for solutions of CuCl2.2H2O in water? Which ion is responsible for the colour that is observed in the CuCl2 solution? What is observed when th Na2SO3 solution is added to the CuCl2? Which gas can be smelled when H2SO4 is added to the mixture? What is the colour of the CuCl when it is filtered on Buchner funnel? (before it is exposed to the atmosphere) What is the colour of the CuCl when it is filterd on the Bucher funnel? (before it is exposed to the atmosphere) Does the colour of the CuCl remain the same if you leave it to dry in the atmosphere? Calculate the percentage yields for the experiment
- E0 values for Ce4 + / Ce3 + and Fe2 + / Fe values are +1.72 and - 0.4 V, respectively. What conclusion can you draw from these data?1-Why σ2px lie at higher energy level than π2py and π2pz in N2? 2-Discuss the structure of [Ni (CN)6]-2 on base of VBTGiven A, B, and C… [A] U(VI) as uraninite; UO2 (where Fe2+= reductant; Fe(OH)3 ferrihydrite= product): 2Fe2+ + UO22+ +3H2O + H+ ---- > 2Fe(OH)3 + U4+ +2H2O [B] U(VI) as uraninite; UO2 (where Mn2+= reductant; MnO2 pyrolusite= product): 2H2O + UO22+ + Mn2+ ---- > UO2 + B - MnO2 + 4H+ [C] U(VI) as as uraninite; UO2 (where HS-= reductant; S0= product): UO22+ + Hs- ---- > UO2 + S + H+ QUESTION: Use thermodynamic calculations [use redox potential (Eh)] to predict which of the three reductants below is most favorable at pH 3 ? (1) Fe2+ (2) Mn2+ (3) HS-
- 1. A Cr3+ solution is electrolyzed using a current of0.365 A. What mass of Cr(s) (51.9661) is plated outafter 10 hours?2. When an aqueous solution of CuSO4 is electrolyzedcopper metal is deposited. If a constant current waspassed for 5.00 h and 404 mg of Cu (63.546) metal wasdeposited, what was the current?The solubility of Ag₂CO3 is measured and found to be 3.41×10-2 g/L. Use this information to calculate a Ksp value for silver carbonate. Ksp = ___________ The solubility of CaSO4 is measured and found to be 0.689 g/L. Use this information to calculate a Ksp value for calcium sulfate. Ksp = ___________ The solubility of BaF2 is measured and found to be 1.29 g/L. Use this information to calculate a Ksp value for barium fluoride. Ksp = _________ (The attached picture is an example how to solve the given problems; The example's problem is The solubility of Ag2SO3 is measured and found to be 4.51x10-3 g/L. Use this information to calculate a Ksp value for silver sulfite.)Given: 0.35g NaCl, 0.25 g NaHCO3, 0.15 g KCl & 2 g C6H12O6 are present in 100 mL ORS solution (MW: Na: 23, K: 39, Cl: 35, H: 1, C: 12, O: 16) Calculate the total amount of bicarbonate expressed in mmol/L present in the prepared solution a 90.10 mmol/L b 80.61 mmol/L c 29.76 mmol/L d 60.34 mmol/L e 111.11 mmol/L f 20.27 mmol/L
- How many milliliters of 1.5% alcoholic dimethylglyoxime should be used to provide a36.0% excess with 0.6984 q of steel containing 2.95 wt% Ni? Assume that the densityof the dimethylglyoxime solution is 0.790 g/mL. a. How much Ni sample is present? b. How many moles Ni is present in the sample? c. How much DMG is present?Given: 0.35g NaCl, 0.25 g NaHCO3, 0.15 g KCl & 2 g C6H12O6 are present in 100 mL ORS solution (MW: Na: 23, K: 39, Cl: 35, H: 1, C: 12, O: 16) Calculate the total amount of chloride expressed in mmol/L present in the prepared solution 60.34 mmol/L 90.10 mmol/L a 111.11 mmol/L b 29.76 mmol/L c 80.61 mmol/L d 20.27 mmol/LCalculate the pH of a 0.10M solution of NaHSO4 solution taking into account activity (Ka2 = 1.92 for H2SO4).



