Use the table on the information page to predict if a reaction will occur when Ni(s) and hydrochloric acid(aq) are combined. If a reaction occurs, write a balanced equation for the reaction. Submit Answer Retry Entire Group 9 more group attempts remaining Activity Series of Metals Li к Displace H, from H2O({), steam, or acid Ba Sr Ca Na Mg Al Displace H, from Mn Zn steam or acid Cr Ease of oxidation Fe Ni increases Displace H, from Sn Pb acid Н, Sb Cu Hg Do not displace H2 Ag from H2O({), steam, Pd or acid Pt Au
Use the table on the information page to predict if a reaction will occur when Ni(s) and hydrochloric acid(aq) are combined. If a reaction occurs, write a balanced equation for the reaction. Submit Answer Retry Entire Group 9 more group attempts remaining Activity Series of Metals Li к Displace H, from H2O({), steam, or acid Ba Sr Ca Na Mg Al Displace H, from Mn Zn steam or acid Cr Ease of oxidation Fe Ni increases Displace H, from Sn Pb acid Н, Sb Cu Hg Do not displace H2 Ag from H2O({), steam, Pd or acid Pt Au
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 60A
Related questions
Question
100%
Transcribed Image Text:Use the table on the information page to predict if a reaction will occur when Ni(s) and hydrochloric acid(aq) are combined. If a reaction occurs, write a balanced equation for the reaction.
Submit Answer
Retry Entire Group
9 more group attempts remaining
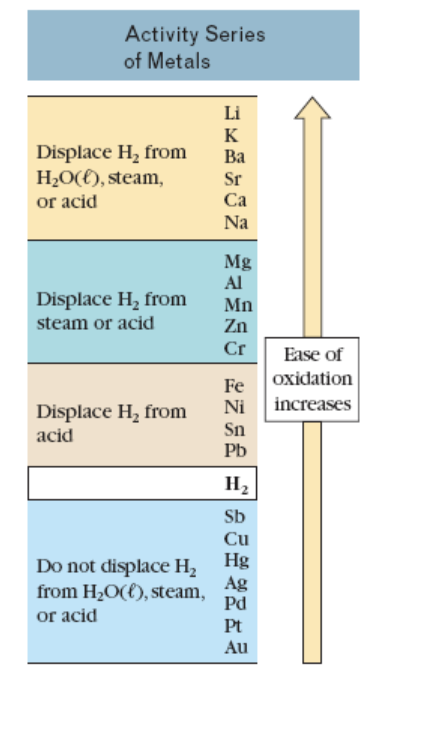
Transcribed Image Text:Activity Series
of Metals
Li
к
Displace H, from
H2O({), steam,
or acid
Ba
Sr
Ca
Na
Mg
Al
Displace H, from
Mn
Zn
steam or acid
Cr
Ease of
oxidation
Fe
Ni
increases
Displace H, from
Sn
Pb
acid
Н,
Sb
Cu
Hg
Do not displace H2
Ag
from H2O({), steam,
Pd
or acid
Pt
Au
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning