9. If you dissolved exactly 100 g of KCl in 100 g of water at 80°C, would the solution be saturated, unsaturated, or supersaturated? 10. If you dissolved 125 g of NaNO3 at 60 °C and then cooled the solution down to 40 °C, what would the solution be considered? Saturated, unsaturated, or supersaturated? 1500
9. If you dissolved exactly 100 g of KCl in 100 g of water at 80°C, would the solution be saturated, unsaturated, or supersaturated? 10. If you dissolved 125 g of NaNO3 at 60 °C and then cooled the solution down to 40 °C, what would the solution be considered? Saturated, unsaturated, or supersaturated? 1500
Chapter80: Crystallization: Purification Of Solids
Section: Chapter Questions
Problem 1P
Related questions
Question
100%
9 and 10
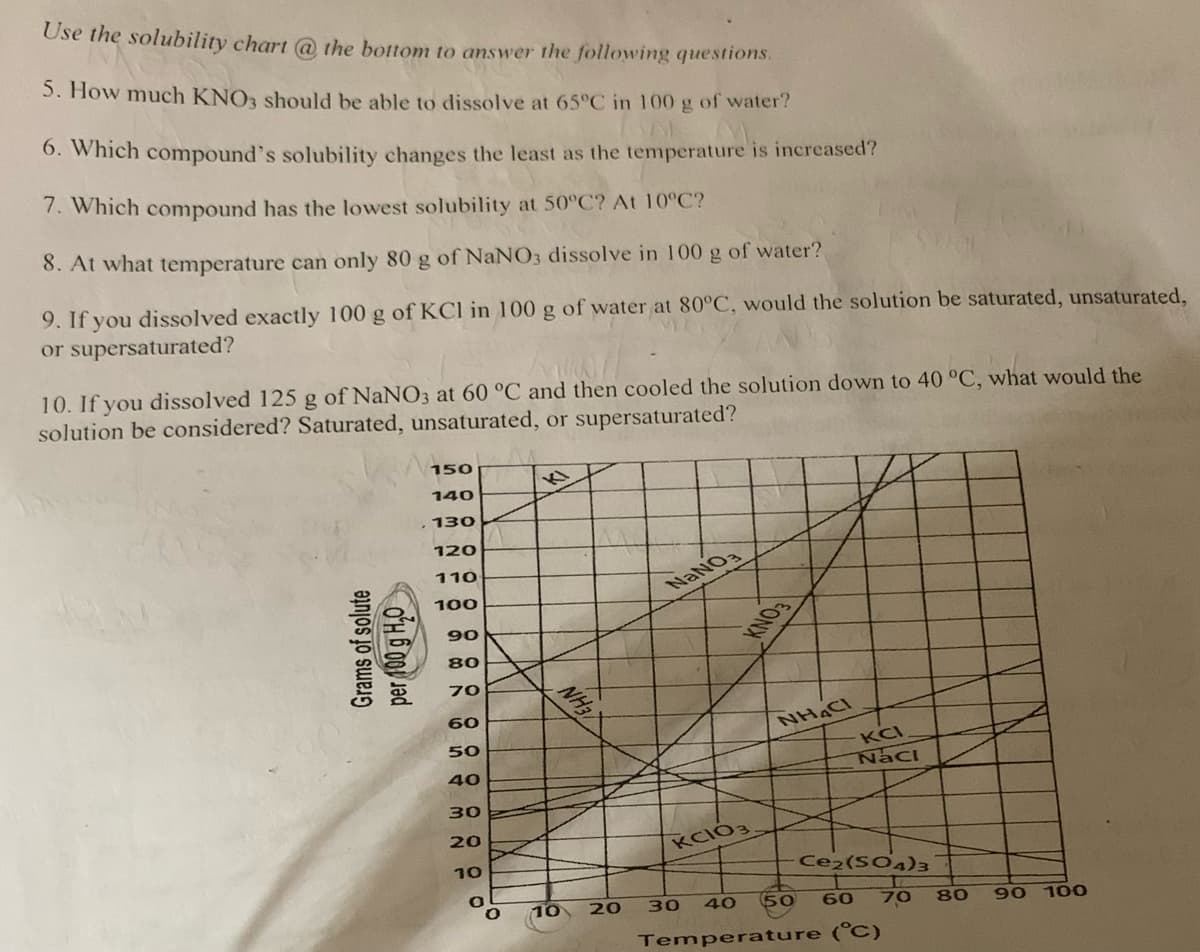
Transcribed Image Text:Use the solubility chart @ the bottom to answer the following questions.
5. How much KNO3 should be able to dissolve at 65°C in 100 g of water?
6. Which compound's solubility changes the least as the temperature is increased?
7. Which compound has the lowest solubility at 50°C? At 10°C?
8. At what temperature can only 80 g of NaNO3 dissolve in 100 g of water?
9. If you dissolved exactly 100 g of KCl in 100 g of water at 80°C, would the solution be saturated, unsaturated,
or supersaturated?
10. If you dissolved 125 g of NaNO3 at 60 °C and then cooled the solution down to 40 °C, what would the
solution be considered? Saturated, unsaturated, or supersaturated?
Grams of solute
per 100 g H₂O
150
140
130
120
110
100
90
80
70
60
50
40
30
20
10
O
NH3
10 20
NaNO3
KCIO3.
NHẠC
30
KCI
Naci
Ce2(SO4)3
50
40
60
Temperature (°C)
70
80
90
100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning