9.6. Formaldehyde may be produced in the reaction between methanol and oxygen: 2CH3OH(1) + O2(g) → 2HCHO(g) + 2H2O(1): AH; = –326.2 kJ The standard heat of combustion of hydrogen is H2(g) +O2(g) → H;O(1): AĤ, = -285.8 kJ/mol (a) Use these heats of reaction and Hess's law to determine the standard heat of the direct decomposition of methanol to form formaldehyde: CH;OH(1) → HCHO(g) + H2(g) (b) Explain why you would probably use the method of Part (a) to determine the heat of the methanol decomposition reaction experimentally rather than carrying out the decomposition reaction and measuring AH, directly.
9.6. Formaldehyde may be produced in the reaction between methanol and oxygen: 2CH3OH(1) + O2(g) → 2HCHO(g) + 2H2O(1): AH; = –326.2 kJ The standard heat of combustion of hydrogen is H2(g) +O2(g) → H;O(1): AĤ, = -285.8 kJ/mol (a) Use these heats of reaction and Hess's law to determine the standard heat of the direct decomposition of methanol to form formaldehyde: CH;OH(1) → HCHO(g) + H2(g) (b) Explain why you would probably use the method of Part (a) to determine the heat of the methanol decomposition reaction experimentally rather than carrying out the decomposition reaction and measuring AH, directly.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.86QE: One of the components of jet engine fuel is n-dodecane, C12H26(), which has a standard enthalpy of...
Related questions
Question
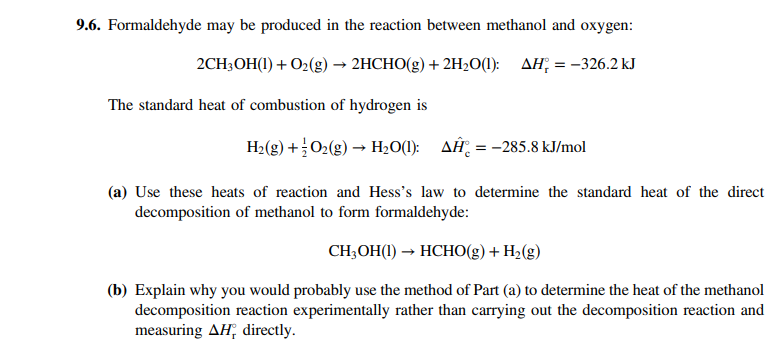
Transcribed Image Text:9.6. Formaldehyde may be produced in the reaction between methanol and oxygen:
2CH3OH(1) + O2(g) → 2HCHO(g) + 2H2O(1): AH; = –326.2 kJ
The standard heat of combustion of hydrogen is
H2(g) +O2(g) → H;O(1): AĤ, = -285.8 kJ/mol
(a) Use these heats of reaction and Hess's law to determine the standard heat of the direct
decomposition of methanol to form formaldehyde:
CH;OH(1) → HCHO(g) + H2(g)
(b) Explain why you would probably use the method of Part (a) to determine the heat of the methanol
decomposition reaction experimentally rather than carrying out the decomposition reaction and
measuring AH, directly.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning