Calculate how much propane C3H3 (in grams) is needed in a combustion reaction to heat 8.00 L of water from 12°C to 100°C (hey it's cold at deer camp) if the burner provides a 50% efficiency for the transfer of the heat energy to the water, and given the following information. A H°f of C3H3 (g) = -103.6 kJ mol-1 A H°f of H20(1) = -285.9 kJ mol-1; A H°f of CO2(g) = -393.5 kJ mol-1; A H°f of 0z is zero Specific heat of water is 4.184 J/g°C Density of water is 1g/ml
Calculate how much propane C3H3 (in grams) is needed in a combustion reaction to heat 8.00 L of water from 12°C to 100°C (hey it's cold at deer camp) if the burner provides a 50% efficiency for the transfer of the heat energy to the water, and given the following information. A H°f of C3H3 (g) = -103.6 kJ mol-1 A H°f of H20(1) = -285.9 kJ mol-1; A H°f of CO2(g) = -393.5 kJ mol-1; A H°f of 0z is zero Specific heat of water is 4.184 J/g°C Density of water is 1g/ml
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section5.7: Enthalpy Calculations
Problem 5.10CYU: Calculate the standard enthalpy of combustion for benzene, C6H6. C6H6() + 15/2 O2(g) 6 CO2(g) + 3...
Related questions
Question
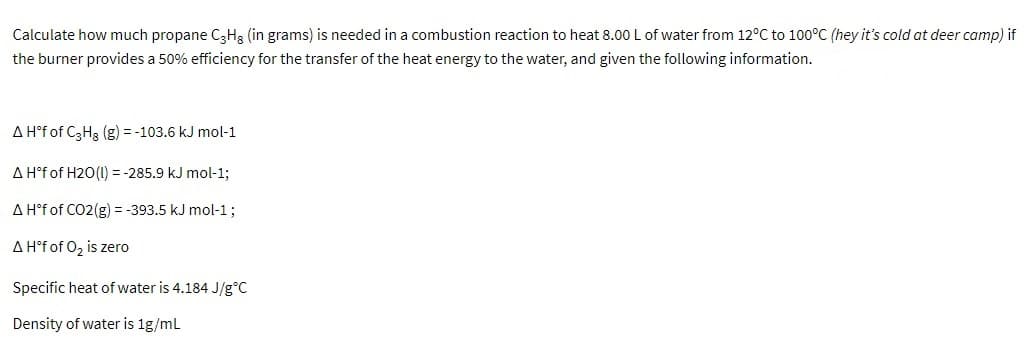
Transcribed Image Text:Calculate how much propane C3H3 (in grams) is needed in a combustion reaction to heat 8.00 L of water from 12°C to 100°C (hey it's cold at deer camp) if
the burner provides a 50% efficiency for the transfer of the heat energy to the water, and given the following information.
A H°f of C3H3 (g) = -103.6 kJ mol-1
A H°f of H20(1) = -285.9 kJ mol-1;
A H°f of CO2(g) = -393.5 kJ mol-1;
A H°f of 0z is zero
Specific heat of water is 4.184 J/g°C
Density of water is 1g/ml
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning