General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.126QP
Related questions
Question
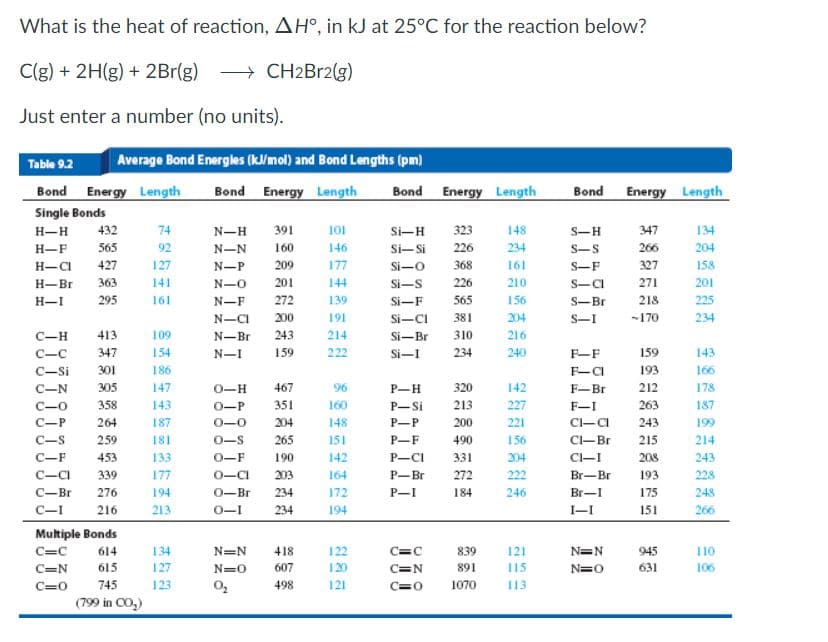
Transcribed Image Text:What is the heat of reaction, AH°, in kJ at 25°C for the reaction below?
C(g) + 2H(g) + 2Br(g) > CH2B12(g)
Just enter a number (no units).
Table 9.2
Average Bond Energles (kl/mol) and Bond Lengths (pm)
Bond Energy Length
Bond Energy Length
Bond Energy Length
Energy Length
Bond
Single Bonds
H-H
432
74
N-H
391
101
Si-H
323
148
S-H
347
134
H-F
565
92
N-N
160
146
Si-Si
226
234
S-S
266
204
H-CI
427
127
N-P
209
177
Si-O
368
161
S-F
327
158
H-Br
363
141
N-0
201
144
Si-S
226
210
S-CI
271
201
H-I
295
161
N-F
272
139
Si-F
565
156
S-Br
218
225
N-CI
200
191
Si-CI
381
204
S-I
-170
234
C-H
413
109
N-Br
243
214
Si-Br
310
216
C-C
347
154
N-I
159
222
Si-I
234
240
F-F
159
143
C-si
301
186
F-a
193
166
C-N
305
147
0-H
467
96
P-H
320
142
F-Br
212
178
C-0
358
143
0-P
351
160
P-Si
213
227
F-I
263
187
C-P
264
187
0-0
204
148
P-P
200
221
CI-CI
243
199
C-S
259
181
0-S
265
151
P-F
490
156
CI-Br
215
214
C-F
453
133
0-F
190
142
P-CI
331
204
CI-I
208
243
C-CI
339
177
0-CI
203
164
P-Br
272
222
Br-Br
193
228
C-Br
276
194
0-Br
234
172
P-I
184
246
Br-I
175
248
C-I
216
213
0-1
234
194
I-I
151
266
Multiple Bonds
C=C
614
134
N=N
418
122
C=c
839
121
N=N
945
110
C=N
615
127
N=0
607
120
C=N
891
115
N=0
631
106
C=0
745
123
498
121
C=0
1070
113
(799 in CO,)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co