9:30 PM Tue Mar 15 22% I ( Lab+8 copy O O Q e Home Insert Draw Layout Review View Table Times New Roman BIUA 习。 31 Additional Problems 1. Balance each of the following reactions: 2. Label each reaction as a synthesis (S), decomposition (D), single displacement (SD), or double displacement (DD) reaction K+ Ch > KCI Li (s) + O (g) > Li;0 (g) Al+ Al:S C:H: + Br > C:H2Bra Sr+ SrO Ca + HO CalOH): + H: Al + HCI > AICI; + H: Zn (s) + HCl (a) > ZnClz (ao + H: Ca(OH)2 (ag) + HCI (ap) > CaClz (ag)+ H:0 ) U+ UF. 3. Predict the products and balance each of the following reactions: a) O (2) > +] b) C:H O (g) > +] c) Na (8) Ch (g) >
9:30 PM Tue Mar 15 22% I ( Lab+8 copy O O Q e Home Insert Draw Layout Review View Table Times New Roman BIUA 习。 31 Additional Problems 1. Balance each of the following reactions: 2. Label each reaction as a synthesis (S), decomposition (D), single displacement (SD), or double displacement (DD) reaction K+ Ch > KCI Li (s) + O (g) > Li;0 (g) Al+ Al:S C:H: + Br > C:H2Bra Sr+ SrO Ca + HO CalOH): + H: Al + HCI > AICI; + H: Zn (s) + HCl (a) > ZnClz (ao + H: Ca(OH)2 (ag) + HCI (ap) > CaClz (ag)+ H:0 ) U+ UF. 3. Predict the products and balance each of the following reactions: a) O (2) > +] b) C:H O (g) > +] c) Na (8) Ch (g) >
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 9RQ: What characterizes an electrolytic cell? What is an ampere? When the current applied to an...
Related questions
Question
100%
Solve it completely otherwise I will downvote
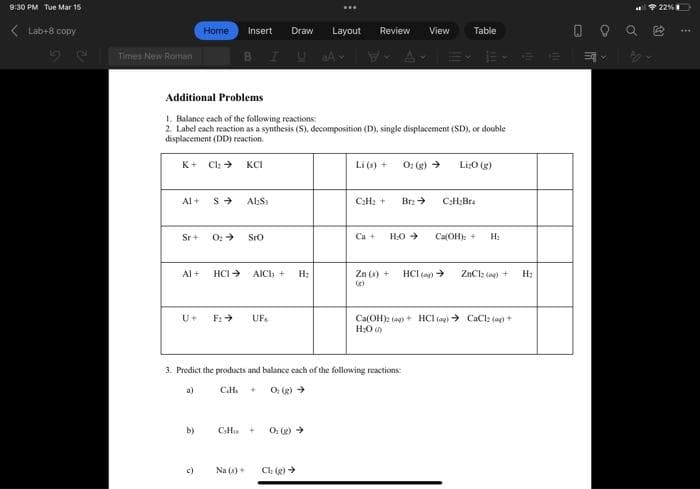
Transcribed Image Text:9:30 PM Tue Mar 15
22% I
( Lab+8 copy
Layout
View
O O Q e
Home
Insert
Draw
Review
Table
Times New Roman
BIUA
31
Additional Problems
1. Balance each of the following reactions:
2. Label each reaction as a synthesis (S), decomposition (D), single displacement (SD), or double
displacement (DD) reaction.
K+ Cla >
KCI
Li (s) +
O: g) >
Li;0 (g)
Al+
Al:S
C2H2 +
Br >
C:H2Bra
Sr+
O: >
SrO
Ca +
HO
CaOH): +
H:
Al +
HCI >
AICI, +
H:
Zn (s) +
HCl (a >
ZnClz (a) +
U+
F2 >
UF.
Ca(OH)2 (ag) + HCI (ay) > CaClz (a)+
H:0
3. Predict the products and balance each of the following reactions:
a)
O (g) >
b)
CH
O (g) >
c)
Na (s)+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax