A 1.0-L 0.010 M buffer with pH 6.50 is given as an assignment to a group of students. Which is the most appropriate weak acid to be used in the buffer preparation? A. Citric Acid B. Acetic Acid C. Carbonic Acid D. Phosphoric Acid What is the concentration of the weak acid and its conjugate base, respectively?
A 1.0-L 0.010 M buffer with pH 6.50 is given as an assignment to a group of students. Which is the most appropriate weak acid to be used in the buffer preparation? A. Citric Acid B. Acetic Acid C. Carbonic Acid D. Phosphoric Acid What is the concentration of the weak acid and its conjugate base, respectively?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter15: Solutions Of Acids And Bases
Section: Chapter Questions
Problem 15.66QE
Related questions
Question
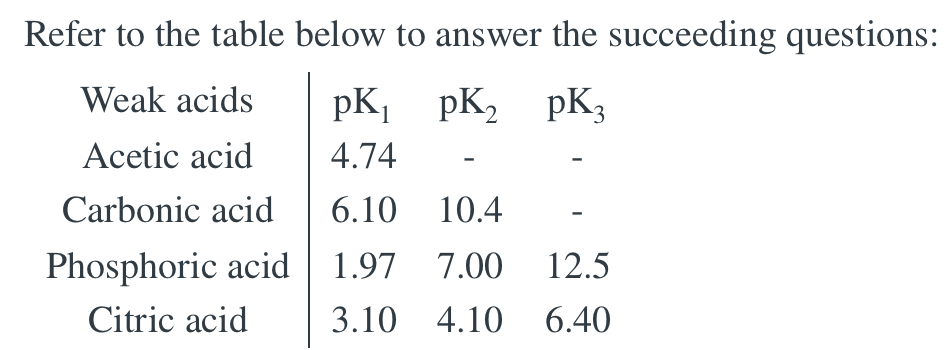
Transcribed Image Text:Refer to the table below to answer the succeeding questions:
Weak acids
pK pK, pK3
Acetic acid
4.74
Carbonic acid
6.10 10.4
Phosphoric acid 1.97 7.00
12.5
Citric acid
3.10 4.10 6.40

Transcribed Image Text:A 1.0-L 0.010 M buffer with pH 6.50 is given as an
assignment to a group of students. Which is the most
appropriate weak acid to be used in the buffer preparation?
A. Citric Acid
B. Acetic Acid
C. Carbonic Acid
D. Phosphoric Acid
What is the concentration of the weak acid and its
conjugate base, respectively?
0.0240 M and 0.0760 M
0.0285 M and 0.0715 M
0.0443 M and 0.0557 M
1.708 × 10¬³ M and 0.0983 M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
