A 1.00-L sample of an unknown gas weighs 0.785 g and is at 0.965 atm and 29.2°C. What is the identity of the gas? Neon Methane Argon Chlorine gas O Carbon Dioxide
A 1.00-L sample of an unknown gas weighs 0.785 g and is at 0.965 atm and 29.2°C. What is the identity of the gas? Neon Methane Argon Chlorine gas O Carbon Dioxide
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter5: Gases
Section: Chapter Questions
Problem 77E: A 15.0-L rigid container was charged with 0.500 atm of krypton gas and 1.50 atm of chlorine gas at...
Related questions
Question
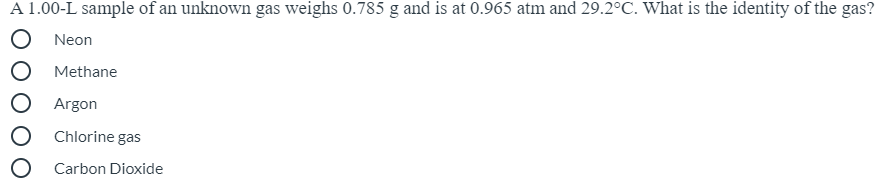
Transcribed Image Text:A 1.00-L sample of an unknown gas weighs 0.785 g and is at 0.965 atm and 29.2°C. What is the identity of the gas?
Neon
Methane
Argon
Chlorine gas
O Carbon Dioxide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning