A 1.00-m solution of acetic acid, CH3COOH, in benzene has a freezing point of 2.96°C. Use the data in the Table to calculate the value of i and suggest an explanation for the unusual result. (Hint: If i is less than 1.0, each formula unit that dissolves yields less than one solute particle, an outcome suggesting aggregation of solute particles.)
A 1.00-m solution of acetic acid, CH3COOH, in benzene has a freezing point of 2.96°C. Use the data in the Table to calculate the value of i and suggest an explanation for the unusual result. (Hint: If i is less than 1.0, each formula unit that dissolves yields less than one solute particle, an outcome suggesting aggregation of solute particles.)
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter7: Equilibria In Multiple-component Systems
Section: Chapter Questions
Problem 7.55E: Determine how ideal the following solutions are by calculating the mole fraction of solute in each...
Related questions
Question
A 1.00-m solution of acetic acid, CH3COOH, in benzene has a freezing point of 2.96°C. Use the data in the Table to calculate the value of i and suggest an explanation for the unusual result. (Hint: If i is less than 1.0, each formula unit that dissolves yields less than one solute particle, an outcome suggesting aggregation of solute particles.)
Answer is:
i = 0.50; formation of dimers of composition (CH3COOH)2, need steps shown to understand though
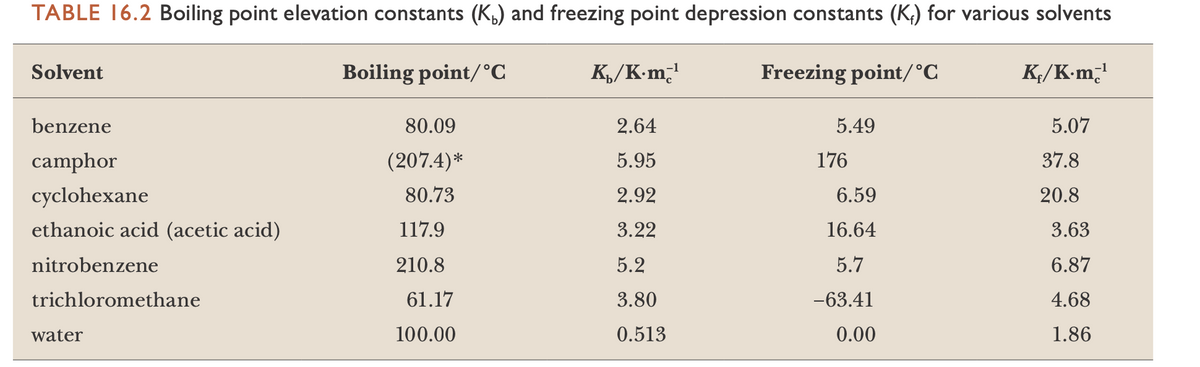
Transcribed Image Text:TABLE 16.2 Boiling point elevation constants (K,) and freezing point depression constants (K;) for various solvents
Solvent
Boiling point/°C
K,/K-m̟'
Freezing point/°C
K;/K-m̟'
benzene
80.09
2.64
5.49
5.07
camphor
(207.4)*
5.95
176
37.8
cyclohexane
80.73
2.92
6.59
20.8
ethanoic acid (acetic acid)
117.9
3.22
16.64
3.63
nitrobenzene
210.8
5.2
5.7
6.87
trichloromethane
61.17
3.80
-63.41
4.68
water
100.00
0.513
0.00
1.86
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,