A 100-W lightbulb is placed in a cylinder equipped with a moveable piston. The lightbulb is turned on for 0.020 h, and the assembly expands from an initial volume of 0.90 L to a final volume of 5.88 L against an external pressure of 1.0 atm. You may want to reference (Pages 370 - 373) Section 9.3 while completing this problem. Part A Use the wattage of the lightbulb and the time it is on to calculate the heat transferred, q, in joules. (Assume that the cylinder and lightbulb assembly comprise the system and assume two significant figures.) Express your answer using two significant figures. Hνα ΑΣφ q = 7700 J Previous Answers Request Answer Submit X Incorrect; Try Again; One attempt remaining
A 100-W lightbulb is placed in a cylinder equipped with a moveable piston. The lightbulb is turned on for 0.020 h, and the assembly expands from an initial volume of 0.90 L to a final volume of 5.88 L against an external pressure of 1.0 atm. You may want to reference (Pages 370 - 373) Section 9.3 while completing this problem. Part A Use the wattage of the lightbulb and the time it is on to calculate the heat transferred, q, in joules. (Assume that the cylinder and lightbulb assembly comprise the system and assume two significant figures.) Express your answer using two significant figures. Hνα ΑΣφ q = 7700 J Previous Answers Request Answer Submit X Incorrect; Try Again; One attempt remaining
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.72PAE: 9.72 Although it can be a nuisance when a laptop computer freezes up and needs to be rebooted, we...
Related questions
Question
100%
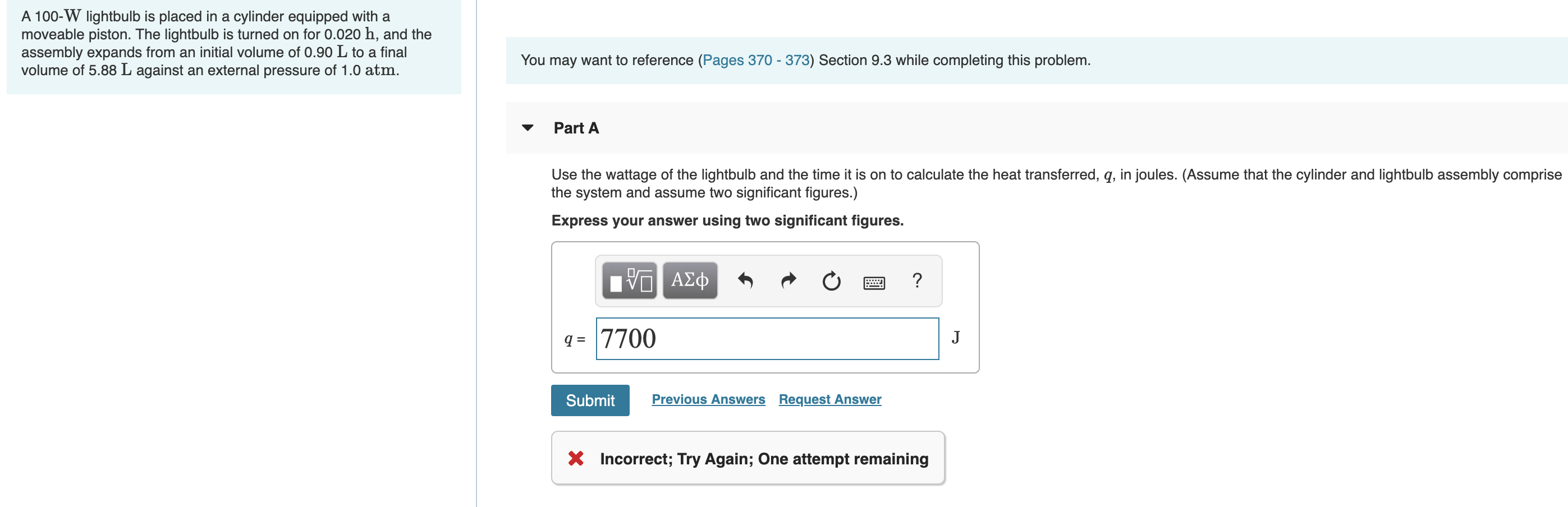
Transcribed Image Text:A 100-W lightbulb is placed in a cylinder equipped with a
moveable piston. The lightbulb is turned on for 0.020 h, and the
assembly expands from an initial volume of 0.90 L to a final
volume of 5.88 L against an external pressure of 1.0 atm.
You may want to reference (Pages 370 - 373) Section 9.3 while completing this problem.
Part A
Use the wattage of the lightbulb and the time it is on to calculate the heat transferred, q, in joules. (Assume that the cylinder and lightbulb assembly comprise
the system and assume two significant figures.)
Express your answer using two significant figures.
Hνα ΑΣφ
q = 7700
J
Previous Answers Request Answer
Submit
X Incorrect; Try Again; One attempt remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning