Chapter9: Aqueous Solutions And Chemical Equilibria
Section: Chapter Questions
Problem 9.29QAP
Related questions
Question
please solve both question. each image has a different question.
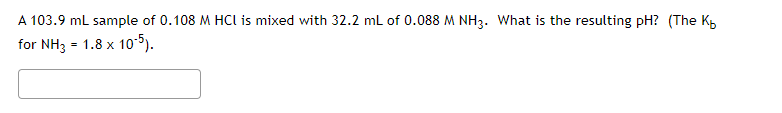
Transcribed Image Text:A 103.9 ml sample of 0.108 M HCI is mixed with 32.2 mL of 0.088 M NH3. What is the resulting pH? (The K
for NH3 = 1.8 x 10-5).
![Calculate the [H*] in a solution that is 0.936 M in NaX and 0.972 M in HX given that the Ka of HX is
8.55 · 10-9. Report your answer in scientific notation to 3 sig figs.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F1390edd2-8754-4537-8acb-2fe3257ca807%2F3f04687b-4b2b-4ae4-b91d-ccb9db66af95%2Fkoxha7_processed.png&w=3840&q=75)
Transcribed Image Text:Calculate the [H*] in a solution that is 0.936 M in NaX and 0.972 M in HX given that the Ka of HX is
8.55 · 10-9. Report your answer in scientific notation to 3 sig figs.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning