A 104.2 mL sample of 1.00 M NaOH is mixed with 52.1 ml of 1.00 MH2SO, in a large Styrofoam coffee cup; the cup is fitted with a lid through which passes a calibrated thermometer. The temperature of each solution before mixing is 23.05°C. After adding the NaOH solution to the coffee cup and stirring the mixed solutions with the thermometer, the maximum temperature measured is 30.90 °C. Assume that the density of the mixed solutions is 1.00 g/mL, that the specific heat of the mixed solutions is 4.18 J/(g°C), and that no heat is lost to the surroundings. 1st attempt Part 11 E See Periodic Table Write a balanced chemical equation for the reaction that takes place in the Styrofoam cup. Remember to include phases in the balanced chemical equation. He (ag). Write a balanced chemical equation for the reaction that takes place in the Styrofoam cup. Remember to include phases in the balanced chemical equation. He Part 2 Is any NaOH or H2SO4 left in the Styrofoam cup when the reaction is over? Choose one: O A. Yes O B. No O See Hir Part 3- Calculate the enthalpy change per mole of H2SO, in the reaction. kJ/mol
A 104.2 mL sample of 1.00 M NaOH is mixed with 52.1 ml of 1.00 MH2SO, in a large Styrofoam coffee cup; the cup is fitted with a lid through which passes a calibrated thermometer. The temperature of each solution before mixing is 23.05°C. After adding the NaOH solution to the coffee cup and stirring the mixed solutions with the thermometer, the maximum temperature measured is 30.90 °C. Assume that the density of the mixed solutions is 1.00 g/mL, that the specific heat of the mixed solutions is 4.18 J/(g°C), and that no heat is lost to the surroundings. 1st attempt Part 11 E See Periodic Table Write a balanced chemical equation for the reaction that takes place in the Styrofoam cup. Remember to include phases in the balanced chemical equation. He (ag). Write a balanced chemical equation for the reaction that takes place in the Styrofoam cup. Remember to include phases in the balanced chemical equation. He Part 2 Is any NaOH or H2SO4 left in the Styrofoam cup when the reaction is over? Choose one: O A. Yes O B. No O See Hir Part 3- Calculate the enthalpy change per mole of H2SO, in the reaction. kJ/mol
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.132QP
Related questions
Question
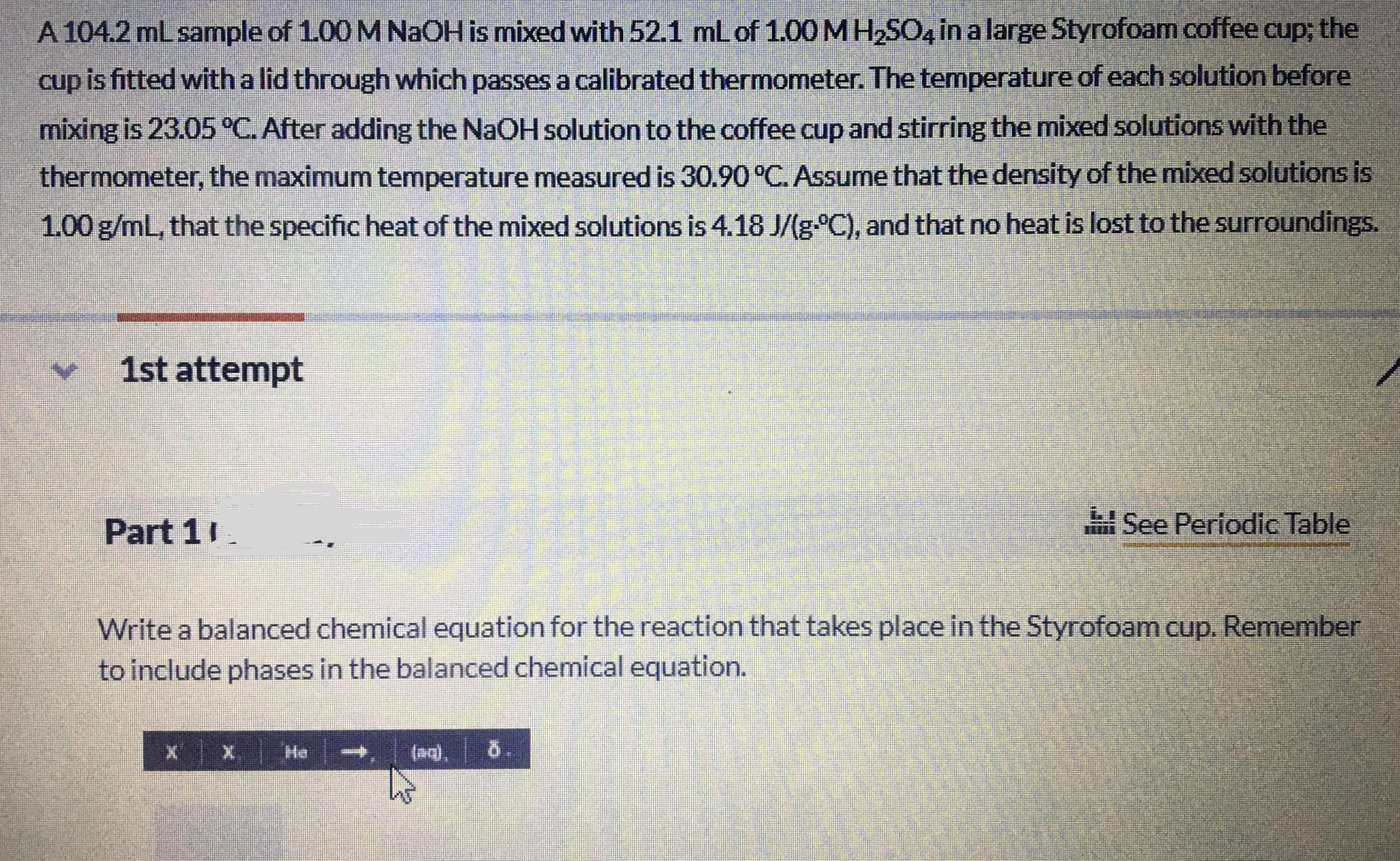
Transcribed Image Text:A 104.2 mL sample of 1.00 M NaOH is mixed with 52.1 ml of 1.00 MH2SO, in a large Styrofoam coffee cup; the
cup is fitted with a lid through which passes a calibrated thermometer. The temperature of each solution before
mixing is 23.05°C. After adding the NaOH solution to the coffee cup and stirring the mixed solutions with the
thermometer, the maximum temperature measured is 30.90 °C. Assume that the density of the mixed solutions is
1.00 g/mL, that the specific heat of the mixed solutions is 4.18 J/(g°C), and that no heat is lost to the surroundings.
1st attempt
Part 11
E See Periodic Table
Write a balanced chemical equation for the reaction that takes place in the Styrofoam cup. Remember
to include phases in the balanced chemical equation.
He
(ag).
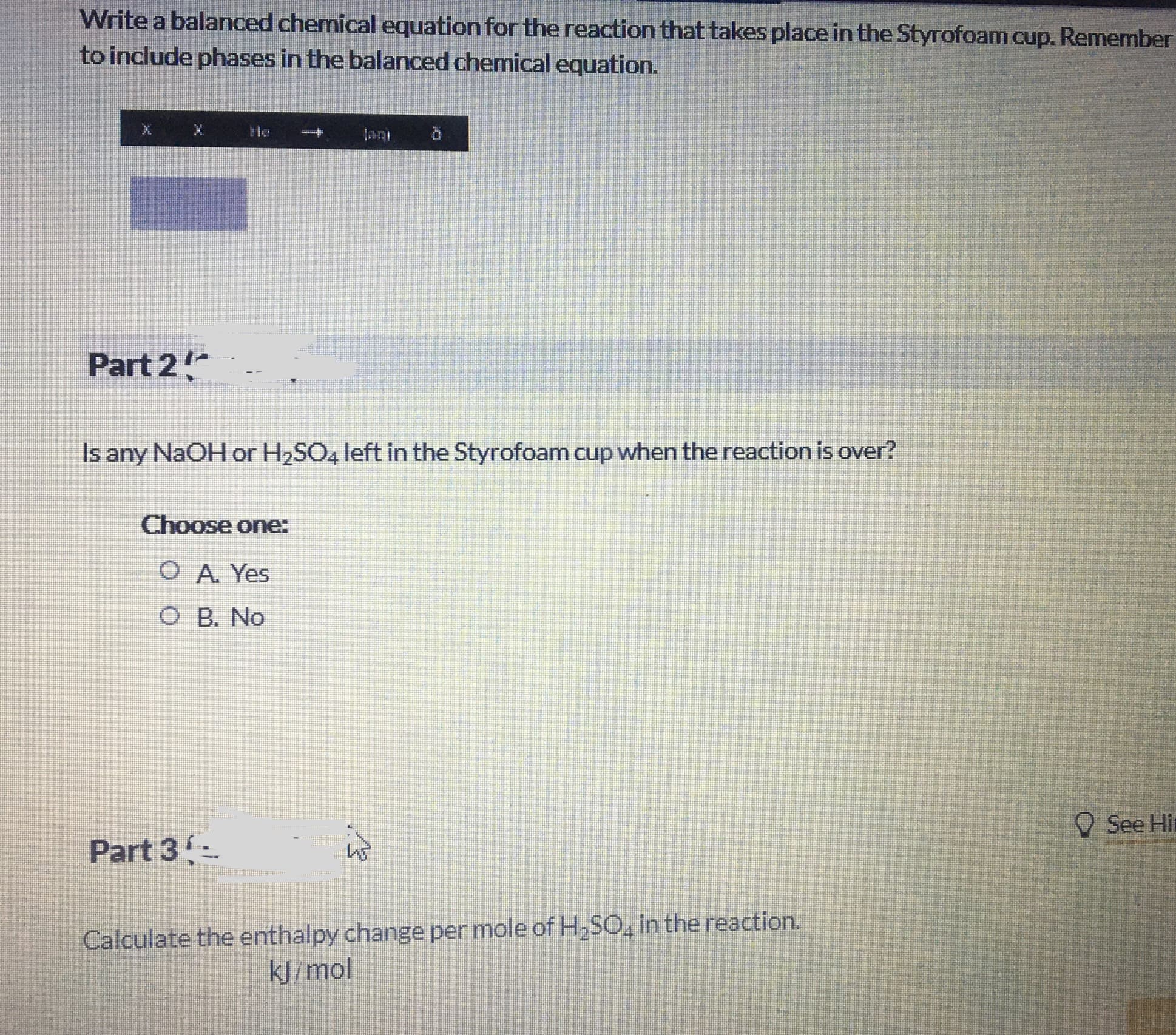
Transcribed Image Text:Write a balanced chemical equation for the reaction that takes place in the Styrofoam cup. Remember
to include phases in the balanced chemical equation.
He
Part 2
Is any NaOH or H2SO4 left in the Styrofoam cup when the reaction is over?
Choose one:
O A. Yes
O B. No
O See Hir
Part 3-
Calculate the enthalpy change per mole of H2SO, in the reaction.
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning