Constants Periodic Table Zinc metal reacts with hydrochloric acid according to the following balanced equation. Zn(s) + 2HC1(aq)→ZnCl2 (aq) + H2 (g) When 0.112 g of Zn(s) is combined with enough HCl to make 51.5 mL of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 22.1 C to 241 C. Part A Find A H for this reaction as written. (Use 1.0 g mL for the density of the solution and 4.18 J/g. C as the specific heat capacity.) AHrxn = kJ/mol Submit Previous Answers Request Answer
Constants Periodic Table Zinc metal reacts with hydrochloric acid according to the following balanced equation. Zn(s) + 2HC1(aq)→ZnCl2 (aq) + H2 (g) When 0.112 g of Zn(s) is combined with enough HCl to make 51.5 mL of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 22.1 C to 241 C. Part A Find A H for this reaction as written. (Use 1.0 g mL for the density of the solution and 4.18 J/g. C as the specific heat capacity.) AHrxn = kJ/mol Submit Previous Answers Request Answer
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.58PAE
Related questions
Question
100%
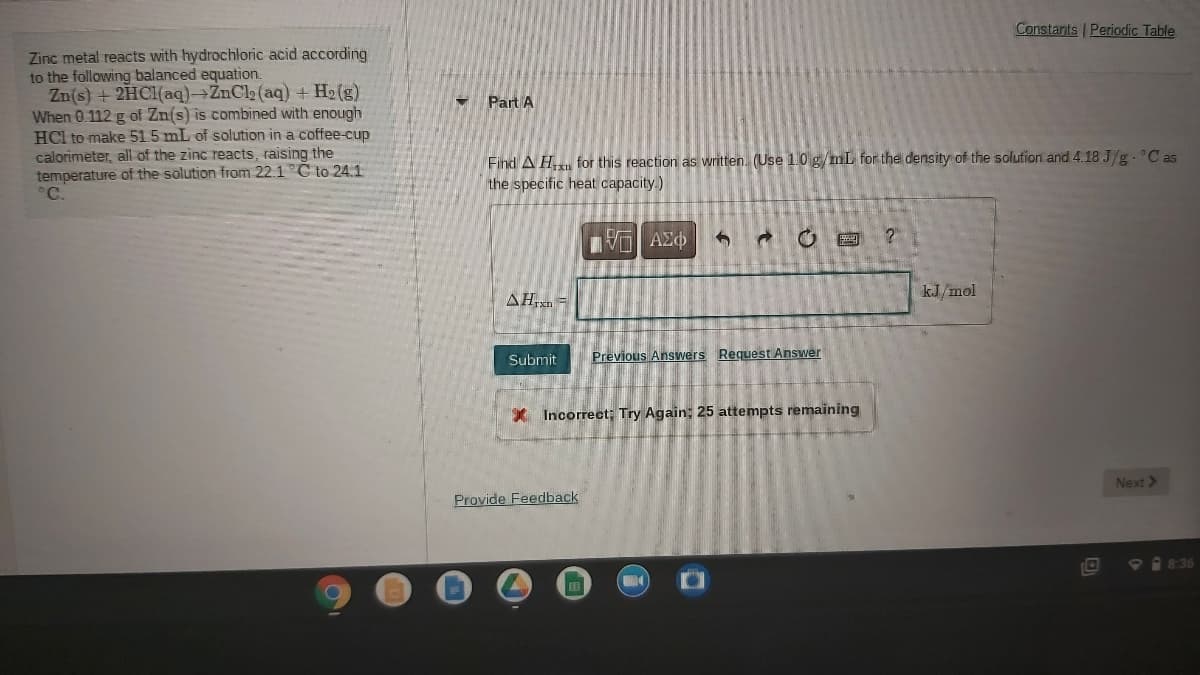
Transcribed Image Text:Constants Periodic Table
Zinc metal reacts with hydrochloric acid according
to the following balanced equation.
Zn(s) + 2HC1(aq)→ZnCl2 (aq) + H2 (g)
When 0.112 g of Zn(s) is combined with enough
HCl to make 515 mL of solution in a coffee-cup
calorimeter, all of the zinc reacts, raising the
temperature of the solution from 22.1 C to 241
°C.
Part A
Find A H for this reaction as written. (Use 1.0 g/mL for the density of the solution and 4.18 J/g. C as
the specific heat capacity.)
να ΑΣφ
AH,xn -
kJ/mol
Submit
Previous Answers Request Answer
* Incorrect; Try Again; 25 attempts remaining
Next >
Provide Feedback
P 8:36
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning