a) 1ne Schrödinger equation for Li²+ is a2 + 87²m ax² ' ay² ™ əz² h? 3e? p = Eµ Briefly explain what each term in the equation represents. b) The wave function for the 3s orbital of Li2+ is P35 (27 – 180 + 20²) exp (–- 729na where o = 3. Calculate the distance (in the unit of ao) of the radial node(s) from the nucleus. Using r as the x-axis, sketch out the diagrams of both 35 and wžs. c) If you were to calculate the distance from the nucleus where maximum probability density occurs (i.e. peaks in the diagrams from part b), what would you do? Briefly describe in words. You do not need to solve for the exact numbers.
a) 1ne Schrödinger equation for Li²+ is a2 + 87²m ax² ' ay² ™ əz² h? 3e? p = Eµ Briefly explain what each term in the equation represents. b) The wave function for the 3s orbital of Li2+ is P35 (27 – 180 + 20²) exp (–- 729na where o = 3. Calculate the distance (in the unit of ao) of the radial node(s) from the nucleus. Using r as the x-axis, sketch out the diagrams of both 35 and wžs. c) If you were to calculate the distance from the nucleus where maximum probability density occurs (i.e. peaks in the diagrams from part b), what would you do? Briefly describe in words. You do not need to solve for the exact numbers.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter6: The Structure Of Atoms
Section: Chapter Questions
Problem 47GQ: Which of the following are applicable when explaining the photoelectric effect? Correct any...
Related questions
Question
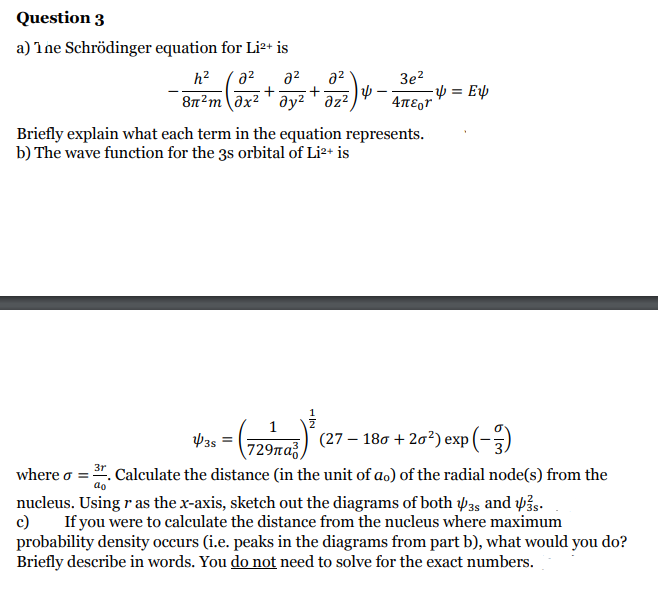
Transcribed Image Text:Question 3
a) Ine Schrödinger equation for Li²+ is
h?
a2
a2
Зе?
8n2m ax2 ' y² ' az²
Briefly explain what each term in the equation represents.
b) The wave function for the 3s orbital of Li2+ is
(729ma) (27 – 180 + 20?) exp (-)
729па3,
where o = . Calculate the distance (in the unit of ao) of the radial node(s) from the
ao
nucleus. Using r as the x-axis, sketch out the diagrams of both 35 and 43s.
If you were to calculate the distance from the nucleus where maximum
c)
probability density occurs (i.e. peaks in the diagrams from part b), what would you do?
Briefly describe in words. You do not need to solve for the exact numbers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning