A 2.5 L tank can withstand a maximum pressure of 800 kPa. Calculate the temperature that the tank will explode, given the tank has a pressure of 180 kPa at 25°C. 32281 Li Be Na Mg Rb Cs Ba Fr Ra Sc Ac above 67.05 K above 1546.8 K below 800.2 K above 135.6 K Zr Nb Mo Hf Rf below 970.4 K 28 O above 1267.2 K 81 E Periodic Table of the Elements Ab T Mn Symbol Co Ni Cu Tc Ru Rh Pd Ag Cd C 11 1 38 F3 SZ Re Os Db Sg Bh Hs Mt Ds Rg Cn Uut "FI Uup "Lv Uus Uuo "Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No OF Ne Si P S Cl Ar As Se Br Kr Sb He Pt Au Hg Tl Pb Bi Po At Rn T
A 2.5 L tank can withstand a maximum pressure of 800 kPa. Calculate the temperature that the tank will explode, given the tank has a pressure of 180 kPa at 25°C. 32281 Li Be Na Mg Rb Cs Ba Fr Ra Sc Ac above 67.05 K above 1546.8 K below 800.2 K above 135.6 K Zr Nb Mo Hf Rf below 970.4 K 28 O above 1267.2 K 81 E Periodic Table of the Elements Ab T Mn Symbol Co Ni Cu Tc Ru Rh Pd Ag Cd C 11 1 38 F3 SZ Re Os Db Sg Bh Hs Mt Ds Rg Cn Uut "FI Uup "Lv Uus Uuo "Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No OF Ne Si P S Cl Ar As Se Br Kr Sb He Pt Au Hg Tl Pb Bi Po At Rn T
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 113QRT
Related questions
Question
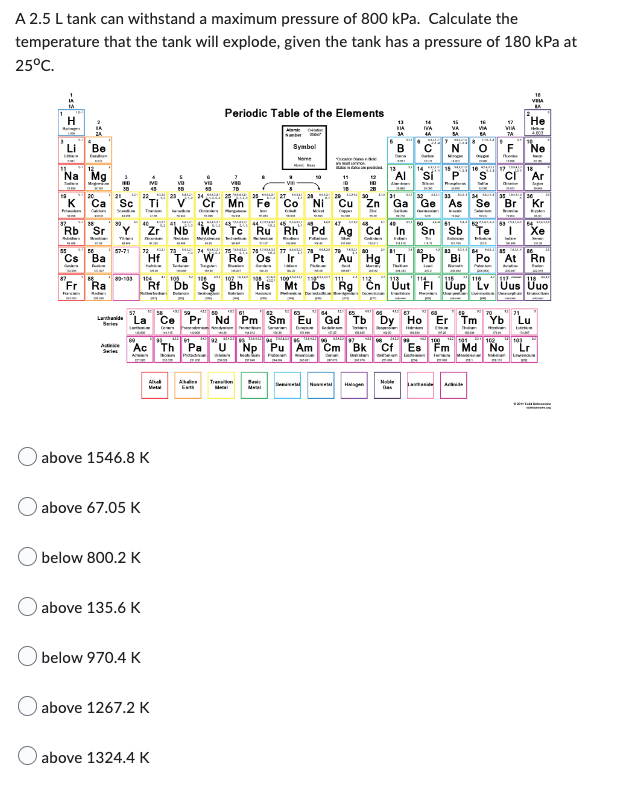
Transcribed Image Text:A 2.5 L tank can withstand a maximum pressure of 800 kPa. Calculate the
temperature that the tank will explode, given the tank has a pressure of 180 kPa at
25°C.
H
Be
Na Mg
K
Rb
Sr
Ba
Fr Ra
Sc Ti
57-71
Lanthanide La Ce
above 67.05 K
Hf
below 800.2 K
above 1546.8 K
above 135.6 K
O below 970.4 K
Viet
va
above 1267.2 K
23 24 25 26 27
Cr Mn
above 1324.4 K
Periodic Table of the Elements
VID
75
Symbol
Abaline
Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb
Tra
Fe Co Ni Cu
Mesa
13
29 30 31
Pt Au Hg
13
HA
Re
89-103 104
106
112 118
114
Rf Db Sg Bh Hs Mt Ds Rg Cn Uut "FI Uup "Lv Uus Uuo
Nawal
B
33
Zn Ga Ge As
14
Ativice "Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No
Series
W
Moham
www.m
MINN
N
Bas
16
VIA
34
Lavand
17
VIA
7A
35
Se Br
71
Pr Nd
Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
as
18
VELA
BA
He
Ne
Xe
At Rn
36
Kr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning