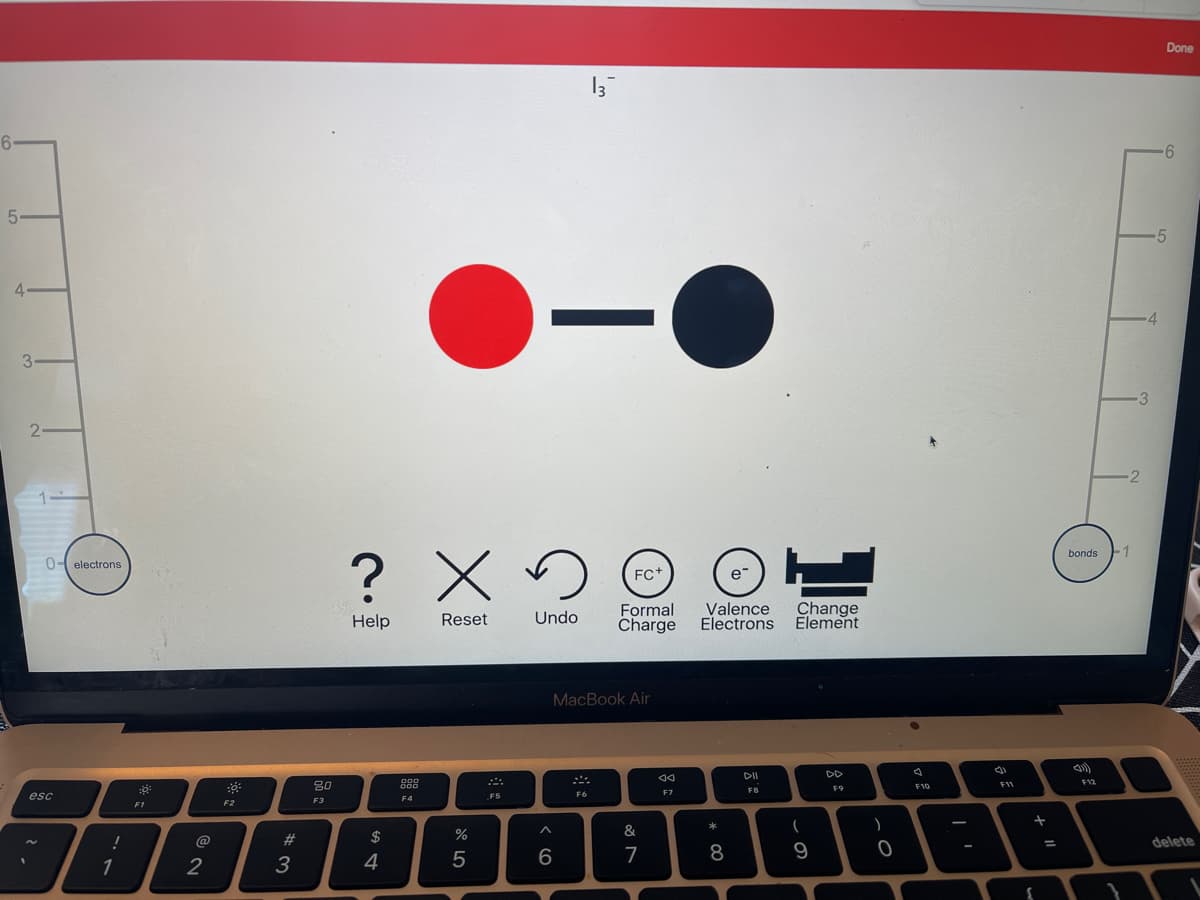
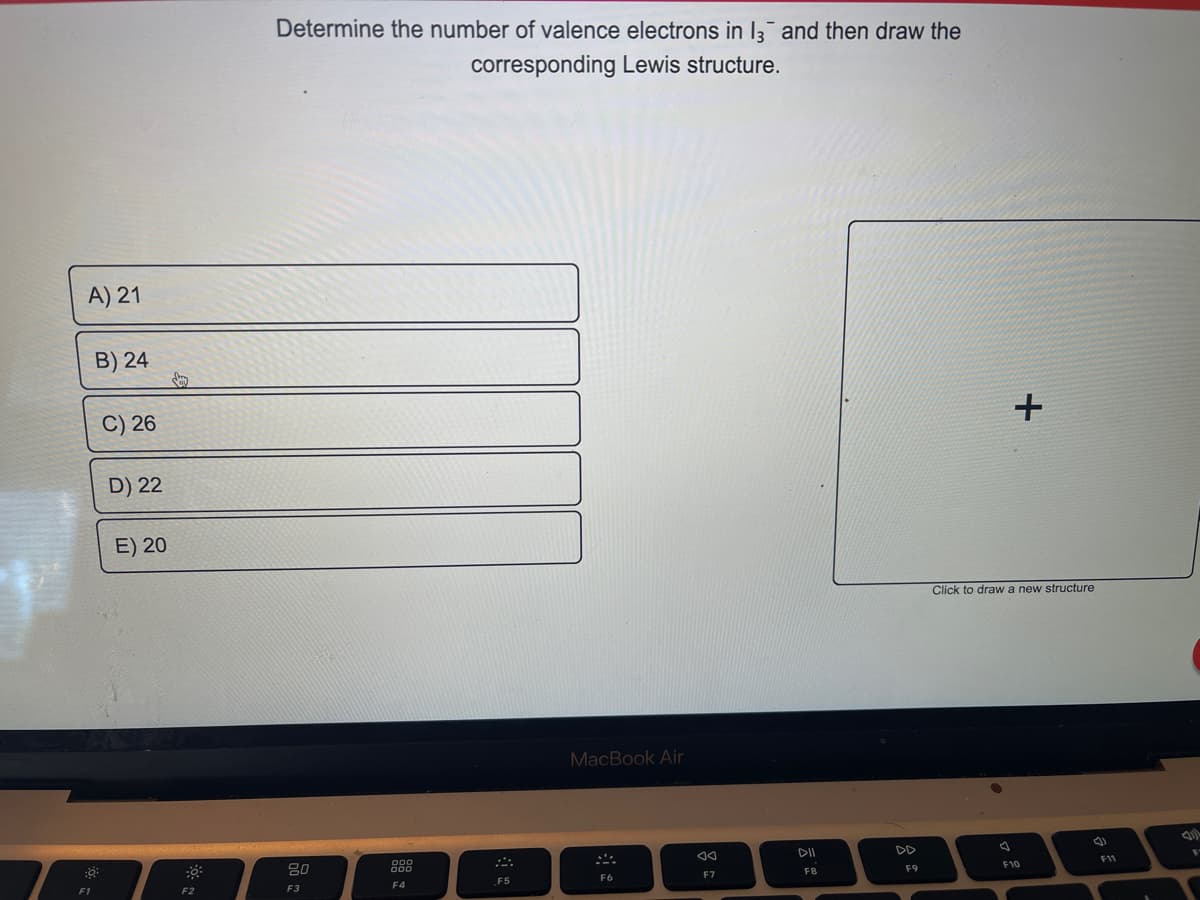
A) 21 B) 24 C) 26 D) 22 E) 20 Determine the number of valence electrons in 13 and then draw the corresponding Lewis structure. + Click to draw a new structure
A) 21 B) 24 C) 26 D) 22 E) 20 Determine the number of valence electrons in 13 and then draw the corresponding Lewis structure. + Click to draw a new structure
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter2: Lewis Structures
Section: Chapter Questions
Problem 13E: Carbon monoxide (CO) is an example of an overall neutral molecule (netcharge=0) that hasnon-zero...
Related questions
Question
100%
Transcribed Image Text:6-
5-
4-
3-
1
2
0-electrons
esc
!
1
::
F1
2
38:
F2
#
3
80
F3
? X
Help
Reset
$
4
000
000
F4
675
%
F5
-
Undo
13
^
6
MacBook Air
F6
FC+
Formal Valence Change
Charge Electrons Element
&
7
:8
F7
*
e¯
8
DII
F8
(
9
DD
F9
)
0
F10
31
F11
+
bonds -1
F12
3
2
4
Done
6
-5
delete
Transcribed Image Text:A) 21
B) 24
:0³:
F1
C) 26
D) 22
E) 20
S
F2
Determine the number of valence electrons in 13 and then draw the
corresponding Lewis structure.
80
F3
200
F4
F5
MacBook Air
F6
JA
F7
DII
F8
F9
+
Click to draw a new structure
B
F10
F11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning