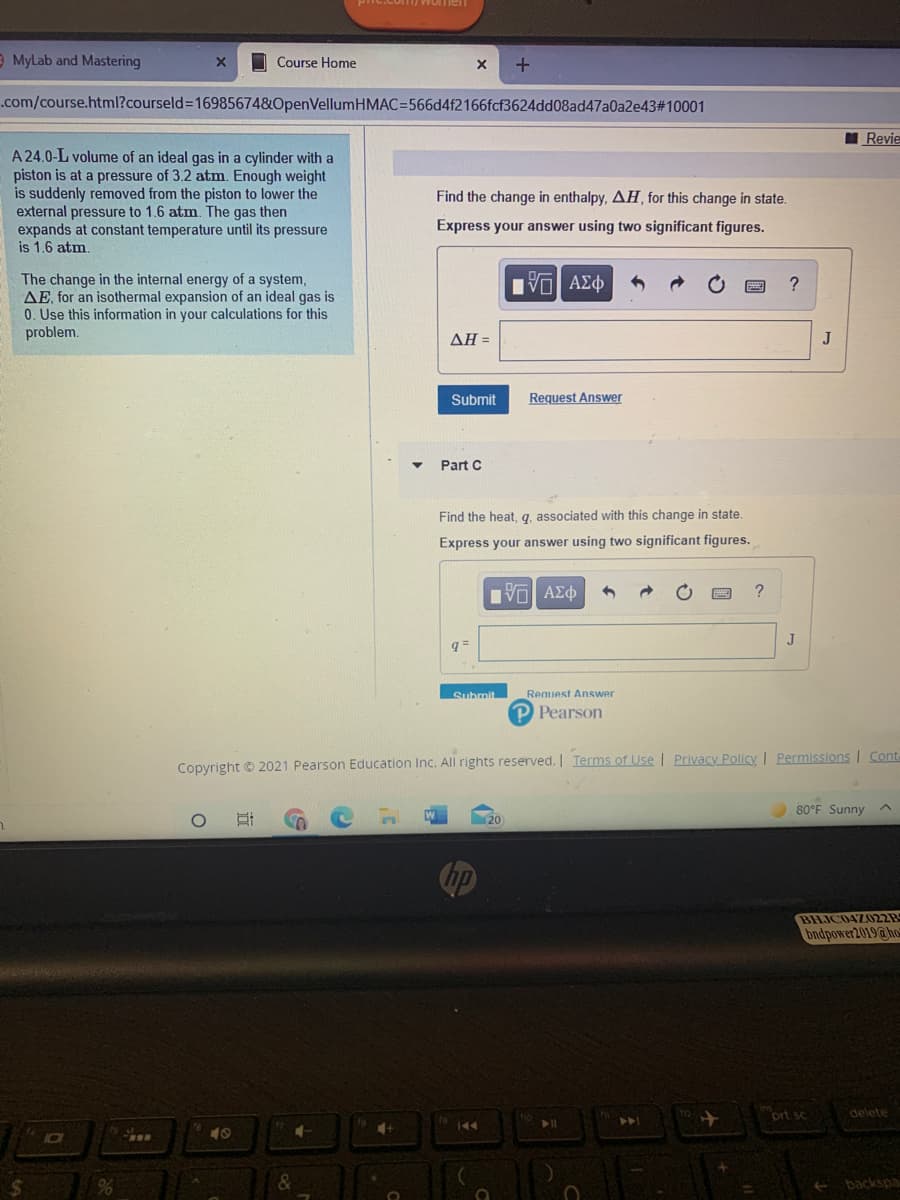
A 24.0-L volume of an ideal gas in a cylinder with a piston is at a pressure of 3.2 atm. Enough weight is suddenly removed from the piston to lower the external pressure to 1.6 atm. The gas then expands at constant temperature until its pressure is 1.6 atm. The change in the internal energy of a system, AE, for an isothermal expansion of an ideal gas is 0. Use this information in your calculations for this problem.
A 24.0-L volume of an ideal gas in a cylinder with a piston is at a pressure of 3.2 atm. Enough weight is suddenly removed from the piston to lower the external pressure to 1.6 atm. The gas then expands at constant temperature until its pressure is 1.6 atm. The change in the internal energy of a system, AE, for an isothermal expansion of an ideal gas is 0. Use this information in your calculations for this problem.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 122QRT
Related questions
Question
Transcribed Image Text:Women
E MyLab and Mastering
O Course Home
.com/course.html?courseld3D16985674&OpenVellumHMAC=566d4f2166fcf3624dd08ad47a0a2e43#10001
Revie
A 24.0-L volume of an ideal gas in a cylinder with a
piston is at a pressure of 3.2 atm. Enough weight
is suddenly removed from the piston to lower the
external pressure to 1.6 atm. The gas then
expands at constant temperature until its pressure
is 1.6 atm.
Find the change in enthalpy, AH, for this change in state.
Express your answer using two significant figures.
The change in the internal energy of a system,
AE, for an isothermal expansion of an ideal gas is
0. Use this information in your calculations for this
problem.
?
ΔΗΞ
J
Submit
Request Answer
Part C
Find the heat, q, associated with this change in state.
Express your answer using two significant figures.
?
J
q =
Submit
Request Answer
P Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy Permissions | Cont
80°F Sunny
BHJC04Z022B
bndpower2019@ ho
ort sc
delete
144
40
backspa
近
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning