A 24.2 mL sample of 0.356 M methylamine , CH3NH, , is titrated with 0.303 M nitric acid. (1) Before the addition of any nitric acid , the pH is| (2) After adding 12.0 mL of nitric acid, the pH is (3) At the titration midpoint, the pH is | (4) At the equivalence point, the pH is [ (5) After adding 44.4 mL of nitric acid , the pH is
A 24.2 mL sample of 0.356 M methylamine , CH3NH, , is titrated with 0.303 M nitric acid. (1) Before the addition of any nitric acid , the pH is| (2) After adding 12.0 mL of nitric acid, the pH is (3) At the titration midpoint, the pH is | (4) At the equivalence point, the pH is [ (5) After adding 44.4 mL of nitric acid , the pH is
Chapter7: Neutralization Titrations And Graphical Representations
Section: Chapter Questions
Problem 1P
Related questions
Question
100%
Plzz answer as soon as possible..
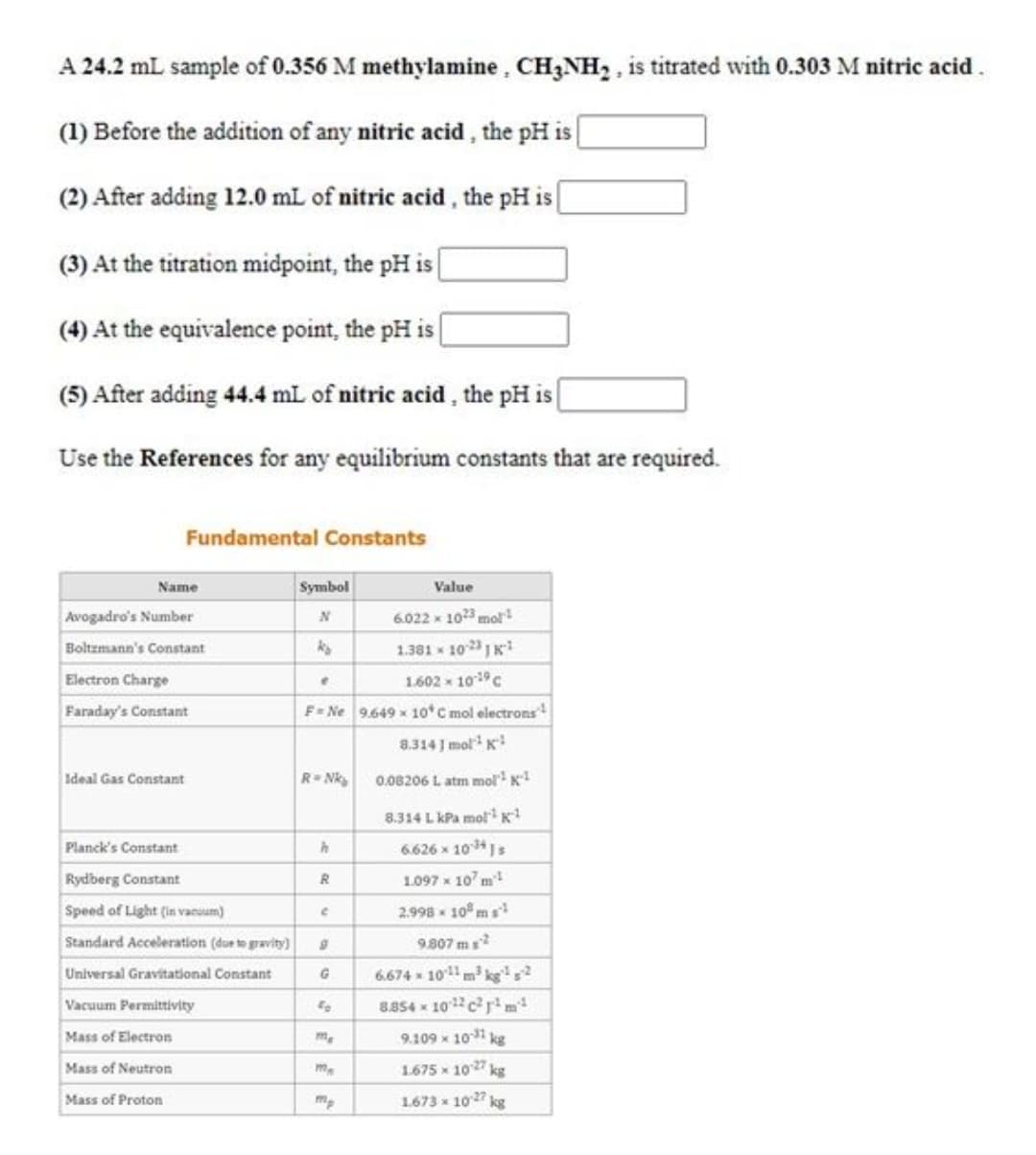
Transcribed Image Text:A 24.2 mL sample of 0.356 M methylamine, CH3NH, , is titrated with 0.303 M nitric acid.
(1) Before the addition of any nitric acid , the pH is|
(2) After adding 12.0 mL of nitric acid, the pH is
(3) At the titration midpoint, the pH is
(4) At the equivalence point, the pH is [
(5) After adding 44.4 mL of nitric acid , the pH is
Use the References for any equilibrium constants that are required.
Fundamental Constants
Name
Symbol
Value
Avogadro's Number
6.022 x 1023 mol
Boltzmann's Constant
1.381 - 102 1Ki
Electron Charge
1.602 x 1019C
Faraday's Constant
F= Ne 9.649 x 10 C mol electrons
8.314 J mol K
Ideal Gas Constant
R Nk
0.08206 L atm molK
8.314 L kPa moK
Planck's Constant
6.626 x 101s
Rydberg Constant
1097 x 10' m!
Speed of Light (in vacuum)
2.998 - 10 ms
Standard Acceleration (due te gravity)
9.807 m
6.674 10m kg'?
8.854 x 102cr m
Universal Gravitational Constant
Vacuum Permittvity
40
Mass of Electron
9.109 x 10 kg
Mass of Neutron
1675 x 1027 kg
Mass of Proton
1.673 x 1027 kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning