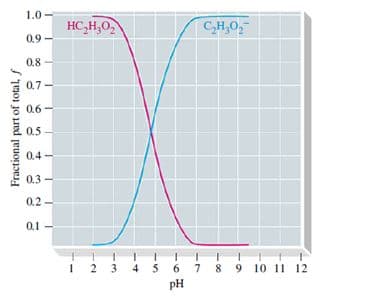
The graph below, which is related to a titration curve, shows the fraction (f) of the stoichiometric amount of acetic acid present as non-ionized CH3COOH and as acetate ion, CH3COO-, as a function of the pH of the solution containing these species. (Graph piture attached) Explain the significance of the point at which the two curves cross. What are the fractions and the pH at that point?
The graph below, which is related to a titration curve, shows the fraction (f) of the stoichiometric amount of acetic acid present as non-ionized CH3COOH and as acetate ion, CH3COO-, as a function of the pH of the solution containing these species. (Graph piture attached) Explain the significance of the point at which the two curves cross. What are the fractions and the pH at that point?
Chapter3: Mechanisms
Section: Chapter Questions
Problem 143EQ
Related questions
Question
The graph below, which is related to a titration curve, shows the fraction (f) of the stoichiometric amount of acetic acid present as non-ionized CH3COOH and as acetate ion, CH3COO-, as a function of the pH of the solution containing these species.
(Graph piture attached)
Explain the significance of the point at which the two curves cross. What are the fractions and the pH at that point?
Transcribed Image Text:1.0-
HC,H,O,
CH,0,
0.9
0.8
0.7
0.6 -
0.5
0.4 -
0.3 -
0.2 -
0.1 -
1.
1 2 3 4 5
6 7
8 9 10 11 12
pH
Fractional part of total, f
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning