A 4.09 g sample of calcium sulfide was decomposed into its constituent elements, producing 2.27 g of calcium and 1.82 g of sulfur. Which of the statements are consistent with the law of constant composition (definite proportions)? The mass ratio of S to Ca in every sample of calcium sulfide is 0.80. The ratio of calcium to sulfur will vary based on how the sample was prepared. Every sample of calcium sulfide will have 44.4% mass of sulfur. Every sample of calcium sulfide will have 2.27 g of calcium. The mass percentage of calcium plus the mass percentage of sulfur in every sample of calcium sulfide equals 100%.
A 4.09 g sample of calcium sulfide was decomposed into its constituent elements, producing 2.27 g of calcium and 1.82 g of sulfur. Which of the statements are consistent with the law of constant composition (definite proportions)? The mass ratio of S to Ca in every sample of calcium sulfide is 0.80. The ratio of calcium to sulfur will vary based on how the sample was prepared. Every sample of calcium sulfide will have 44.4% mass of sulfur. Every sample of calcium sulfide will have 2.27 g of calcium. The mass percentage of calcium plus the mass percentage of sulfur in every sample of calcium sulfide equals 100%.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 125CP: Each of the following statements is true, but Dalton might have had trouble explaining some of them...
Related questions
Question
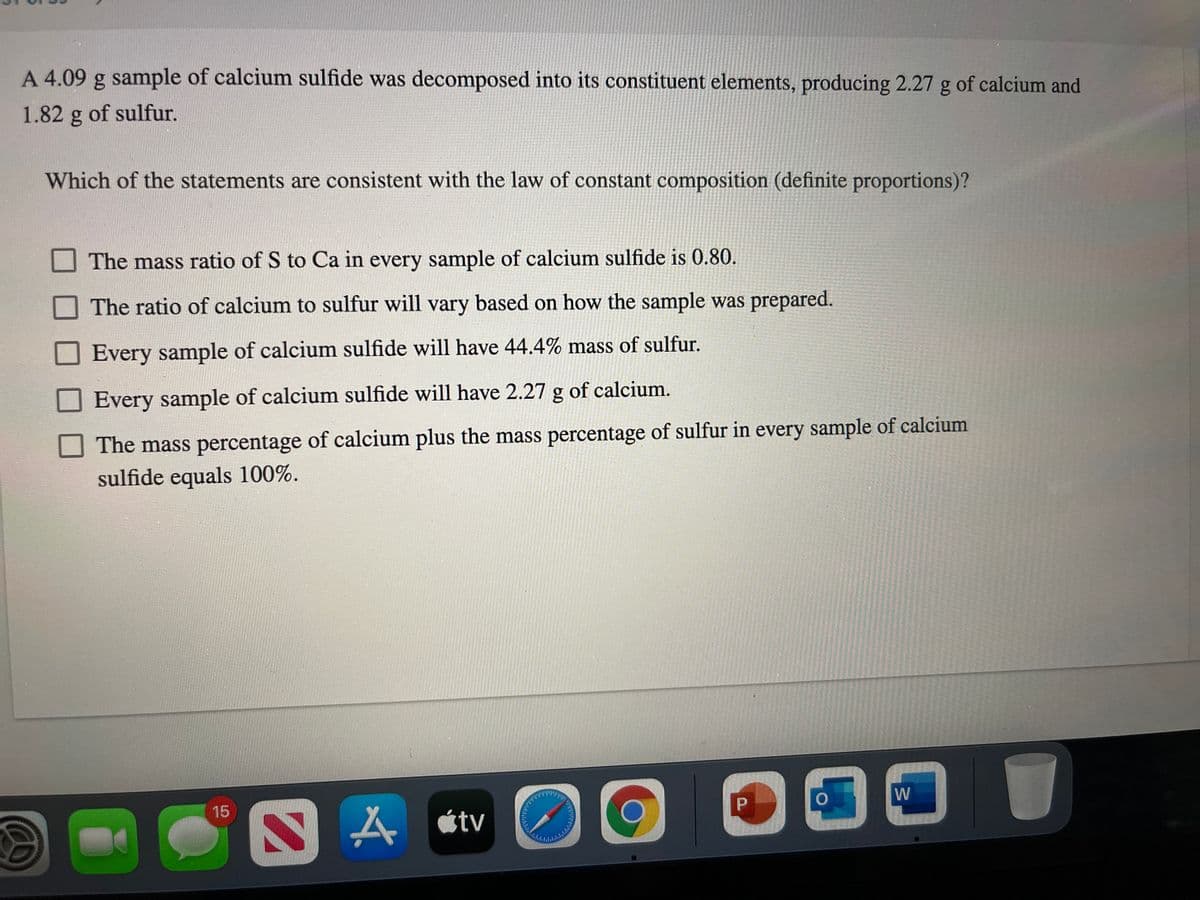
Transcribed Image Text:A 4.09 g sample of calcium sulfide was decomposed into its constituent elements, producing 2.27 g of calcium and
1.82 g of sulfur.
Which of the statements are consistent with the law of constant composition (definite proportions)?
The mass ratio of S to Ca in every sample of calcium sulfide is 0.80.
The ratio of calcium to sulfur will vary based on how the sample was prepared.
Every sample of calcium sulfide will have 44.4% mass of sulfur.
Every sample of calcium sulfide will have 2.27 g of calcium.
The mass percentage of calcium plus the mass percentage of sulfur in every sample of calcium
sulfide equals 100%.
W
15
étv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning