A 45.0 g block of metal at 99.5 °C was dropped into 150.0 g of water at 24.00 °C. The temperature of the mixture rose to 26.40 °C. The specific heat of water is 4.184 J/g°C. Determine the specific heat of the metal. tor initially
A 45.0 g block of metal at 99.5 °C was dropped into 150.0 g of water at 24.00 °C. The temperature of the mixture rose to 26.40 °C. The specific heat of water is 4.184 J/g°C. Determine the specific heat of the metal. tor initially
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 104SCQ: Prepare a graph of specific heat capacities for metals versus their atomic weights. Combine the data...
Related questions
Question
i dont think im on the right track for question 2
![1. The specific heat of aluminum is 0.900 J/°C g.
Problems:
The specific heat of aluminum is 0.900 J/°C g.
d. How much heat energy is needed to raise the temperature of a 8.50 x 102 g block of aluminum
from 22.8 °C to 94.6 °C?
b. Calculate the molar heat capacity of aluminum.
A 45.0 g block of metal at 99.5 °C was dropped into 150.0 g of water at 24.00 °C. The temperature
of the mixture rose to 26.40 °C. The specific heat of water is 4.184 J/g°C. Determine the specific
heat of the metal.
A 200 g piece of lead (0.0305 cal/g K) is heated to 90 °C and dropped into 500 g of water, initially
at 20 °C. Neglecting the heat capacity of the container, find the final temperature of the lead and
water. (H2O: 1.00 cal/g-K)
4.
A 30 g piece of ice, falling off the space shuttle through the Earth's atmosphere, reached a cons
terminal velocity of 8 m/s. Assuming no heat is lost to the surroundings, how far will it have to
in order for all the ice to be melted before it hits the ground? The latent heat of fusion for ice i
80 cal/g.
[Ans: 34 km]
5.
When steam condenses to liquid water, 2.26 kJ of heat is released per gram. The heat from
of air at normal pressure is 1.015 J/g.°C. What is the change in air temperature, assuming
of the air in the room, in g/cr
of steam is used to heat a room containing 6.44 x 104 g of air (20 ft x 12 ft x 8 ft). The specit
2.
3.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fede9f81e-bf87-4602-afc8-0ad2bfc3eb50%2F64946c76-7fb7-4ab3-bb99-3f6dfb665a93%2F83kk15k_processed.jpeg&w=3840&q=75)
Transcribed Image Text:1. The specific heat of aluminum is 0.900 J/°C g.
Problems:
The specific heat of aluminum is 0.900 J/°C g.
d. How much heat energy is needed to raise the temperature of a 8.50 x 102 g block of aluminum
from 22.8 °C to 94.6 °C?
b. Calculate the molar heat capacity of aluminum.
A 45.0 g block of metal at 99.5 °C was dropped into 150.0 g of water at 24.00 °C. The temperature
of the mixture rose to 26.40 °C. The specific heat of water is 4.184 J/g°C. Determine the specific
heat of the metal.
A 200 g piece of lead (0.0305 cal/g K) is heated to 90 °C and dropped into 500 g of water, initially
at 20 °C. Neglecting the heat capacity of the container, find the final temperature of the lead and
water. (H2O: 1.00 cal/g-K)
4.
A 30 g piece of ice, falling off the space shuttle through the Earth's atmosphere, reached a cons
terminal velocity of 8 m/s. Assuming no heat is lost to the surroundings, how far will it have to
in order for all the ice to be melted before it hits the ground? The latent heat of fusion for ice i
80 cal/g.
[Ans: 34 km]
5.
When steam condenses to liquid water, 2.26 kJ of heat is released per gram. The heat from
of air at normal pressure is 1.015 J/g.°C. What is the change in air temperature, assuming
of the air in the room, in g/cr
of steam is used to heat a room containing 6.44 x 104 g of air (20 ft x 12 ft x 8 ft). The specit
2.
3.
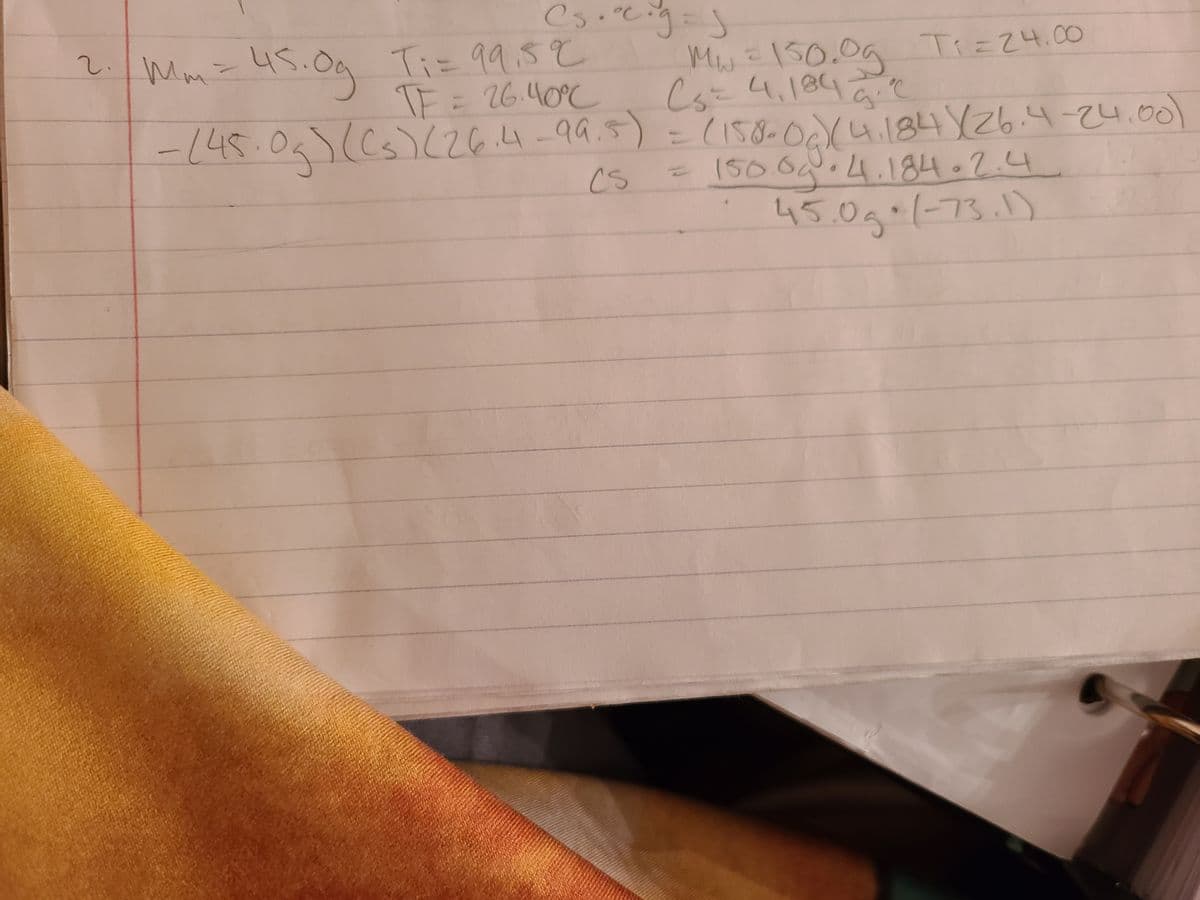
Transcribed Image Text:Cs.Cうこ
て Mm- 4s.0a Tiz 99.5℃
MwE150.0a Ti=Z4.00
Csこ 4.184
TE =
26.40°C
-145.0S((s)c26. )
Cs)(26.4-9a.5
1-99.5)-158-004.184Y26.4-て4.00)
cs
1S06ペ.ム.184 2.4
45.0g-(-73.1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning