A 5.0 ml unknown sample was analyzed by the double indicator method. A single burette was used titration using phenolphthalein and methyl orange respectively and 0.5038 N HCI as the standard solution. Based on the data given: Initial reading of HCI 0.0 mL, Final reading of HCl in Mo is 16.30 mL, Vol. of HCl in Pp is 10.50 and in Mo is 5.80 mL (Answer using the letter of the choices) Identify the compound/s present in the unknown sample. (Choices: A. NaOH & Na2CO3 | B.Na2CO3 C. NaHCO3 | D. NAOH | E. NaHCO3 & Na2CO3 ) Find the weight in grams of the identified compound/s. (Choices: A. 0.0947| B. 0.3097|| C. 0.2730 | D. 0.2730 &0.3097 E. 0.0947 & 0.3097) Find the % composition of the identified compound/s. (Choices: A. 1.89 | B. 1.89 & 6.19 |C. 6.19 | D. 5.24 |E. 5.24 & 6.19)
A 5.0 ml unknown sample was analyzed by the double indicator method. A single burette was used titration using phenolphthalein and methyl orange respectively and 0.5038 N HCI as the standard solution. Based on the data given: Initial reading of HCI 0.0 mL, Final reading of HCl in Mo is 16.30 mL, Vol. of HCl in Pp is 10.50 and in Mo is 5.80 mL (Answer using the letter of the choices) Identify the compound/s present in the unknown sample. (Choices: A. NaOH & Na2CO3 | B.Na2CO3 C. NaHCO3 | D. NAOH | E. NaHCO3 & Na2CO3 ) Find the weight in grams of the identified compound/s. (Choices: A. 0.0947| B. 0.3097|| C. 0.2730 | D. 0.2730 &0.3097 E. 0.0947 & 0.3097) Find the % composition of the identified compound/s. (Choices: A. 1.89 | B. 1.89 & 6.19 |C. 6.19 | D. 5.24 |E. 5.24 & 6.19)
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter24: The Standardization Of A Basic Solution And The Determination Of The Molar Mass Of An Acid
Section: Chapter Questions
Problem 3ASA: A 0.3012g sample of an unknown monoprotic acid requires 24.13mL of 0.0944MNaOH for neutralization to...
Related questions
Question
5
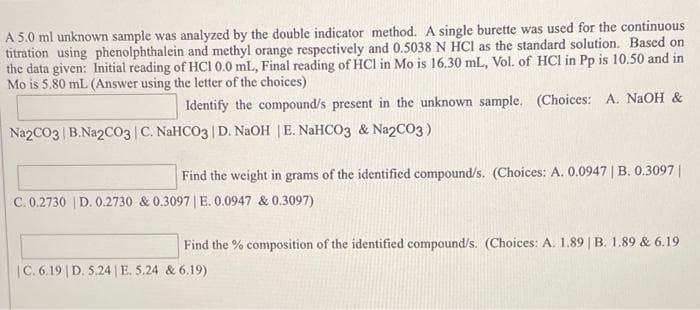
Transcribed Image Text:A 5.0 ml unknown sample was analyzed by the double indicator method. A single burette was used for the continuous
titration using phenolphthalein and methyl orange respectively and 0.5038 N HCI as the standard solution. Based on
the data given: Initial reading of HCI 0.0 mL, Final reading of HCI in Mo is 16.30 mL, Vol. of HCl in Pp is 10.50 and in
Mo is 5.80 mL (Answer using the letter of the choices)
Identify the compound/s present in the unknown sample. (Choices: A. NAOH &
Na2CO3 B.Na2CO03 C. NaHCO3 | D. NaOH |E. NaHCO3 & Na2CO3)
Find the weight in grams of the identified compound/s. (Choices: A. 0.0947 | B. 0.3097||
C. 0.2730 | D. 0.2730 & 0.3097 | E. 0.0947 & 0.3097)
Find the % composition of the identified compound/s. (Choices: A. 1.89 | B. 1.89 & 6.19
|C. 6.19 | D. 5.24 |E. 5.24 & 6.19)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning