A 50 /kg ) is dropped into an insulated tank that contains 0.5 m³ of liquid water at 25 °C (p = 1000 kg/m³, c = 4180 J/kg °C). Determine the temperature when the thermal equilibrium is reached.
A 50 /kg ) is dropped into an insulated tank that contains 0.5 m³ of liquid water at 25 °C (p = 1000 kg/m³, c = 4180 J/kg °C). Determine the temperature when the thermal equilibrium is reached.
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter1: Heat, Temperature, And Pressure
Section: Chapter Questions
Problem 6RQ: One British thermal unit will raise the temperature of _____ 1b of water _____F.
Related questions
Question
سككينلممسس
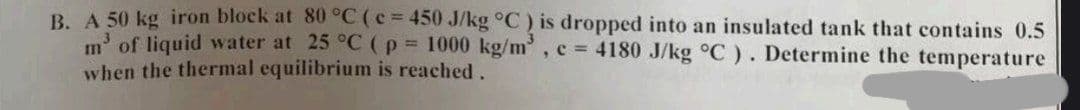
Transcribed Image Text:B. A 50 kg iron block at 80 °C (c = 450 J/kg °C) is dropped into an insulated tank that contains 0.5
m' of liquid water at 25 °C (p = 1000 kg/m³, c = 4180 J/kg °C). Determine the temperature
when the thermal equilibrium is reached.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning