a) 50 mL of CH,CH, Br (bromoethane) and 50 mL of water are poured into a separatory funnel. Bromoethane is a water-insoluble compound with a density of 1.460. The funnel is stoppered and the mixture is shaken vigorously. After standing, two layers separate. Which substance is in which layer? Explain. Bromoethane is more dense than water. The top layer is water and the bottom layer is bromoethane. The top layer is bromoethane and the bottom layer is water. Water is added second. Bromoethane has a higher molar mass than does water.
a) 50 mL of CH,CH, Br (bromoethane) and 50 mL of water are poured into a separatory funnel. Bromoethane is a water-insoluble compound with a density of 1.460. The funnel is stoppered and the mixture is shaken vigorously. After standing, two layers separate. Which substance is in which layer? Explain. Bromoethane is more dense than water. The top layer is water and the bottom layer is bromoethane. The top layer is bromoethane and the bottom layer is water. Water is added second. Bromoethane has a higher molar mass than does water.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 56QAP: Magnesium chloride is an important coagulant used in the preparation of tofu from soy milk. Its...
Related questions
Question
please help with all the parts in this question, it is practice so I want to have all the parts to study

Transcribed Image Text:a) 50 mL of CH, CH, Br (bromoethane) and 50 mL of water are poured into a separatory funnel. Bromoethane is a
water-insoluble compound with a density of 1.460. The funnel is stoppered and the mixture is shaken vigorously. After
ml
standing, two layers separate.
Which substance is in which layer? Explain.
O Bromoethane is more dense than water.
O The top layer is water and the bottom layer is bromoethane.
O The top layer is bromoethane and the bottom layer is water.
O Water is added second.
O Bromoethane has a higher molar mass than does water.
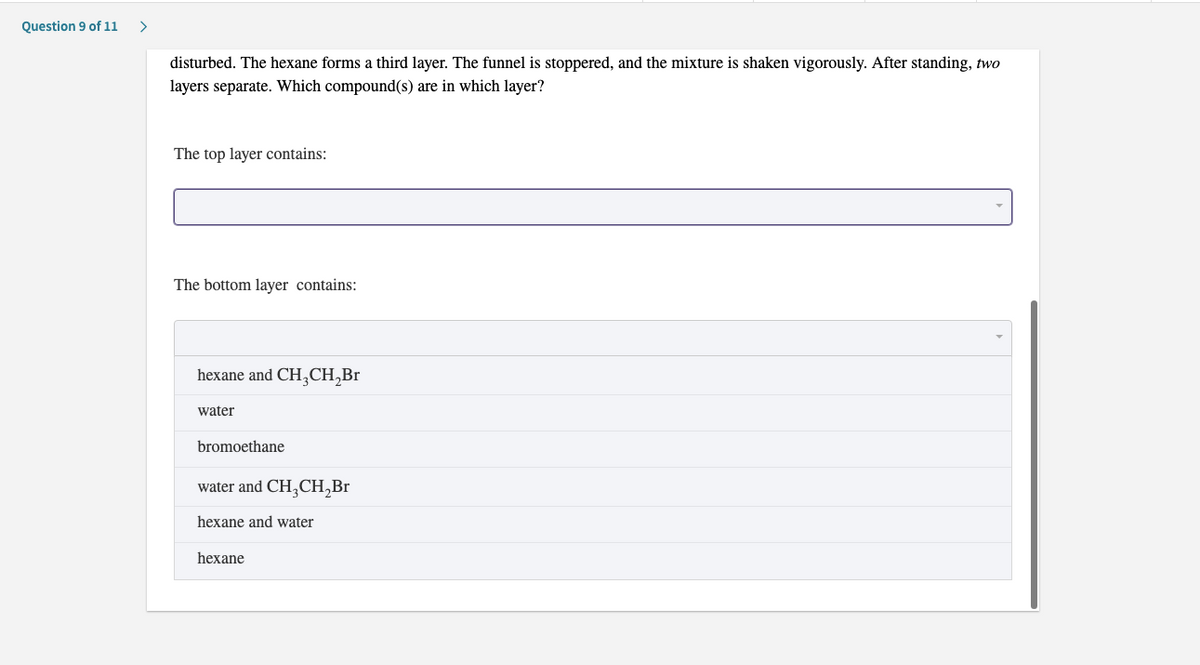
b) Into the same funnel is poured carefully 50 mL of hexane (density = 0.660 g/mL) so that the other two layers are not
disturbed. The hexane forms a third layer. The funnel is stoppered, and the mixture is shaken vigorously. After standing, two
layers separate. Which compound(s) are in which layer?
The top layer contains:
Transcribed Image Text:Question 9 of 11
>
disturbed. The hexane forms a third layer. The funnel is stoppered, and the mixture is shaken vigorously. After standing, two
layers separate. Which compound(s) are in which layer?
The top layer contains:
The bottom layer contains:
hexane and CH,CH,Br
water
bromoethane
water and CH;CH,Br
hexane and water
hexane
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax