Data Set Exp.5 Use the following data to determine the mass percent of the active ingredient in Aspirin a) Preparation of solutions: volume of salicylic acid volume of FECL3 solution SA1 1ml 9ml SA2 3.5ml 6.5ml SA3 6.5ml 3.5ml SA4 1ml 9ml The concentration of salicylic acid= 0.000725M b) Preparation of “STOCK ASA UNKNOWN" The mass of aspirin= 0.135 g The volume of stock solution of aspirin= 100ml The dilution factor for the stock solution= 50/2 Molar mass of SAS = 180.157 g/mole c) Measurements of Absorbance: Solution Absorbance SA1 0.154 SA2 0.524 SA3 0.789 SA4 0.989 The unknown sample 0.259
Data Set Exp.5 Use the following data to determine the mass percent of the active ingredient in Aspirin a) Preparation of solutions: volume of salicylic acid volume of FECL3 solution SA1 1ml 9ml SA2 3.5ml 6.5ml SA3 6.5ml 3.5ml SA4 1ml 9ml The concentration of salicylic acid= 0.000725M b) Preparation of “STOCK ASA UNKNOWN" The mass of aspirin= 0.135 g The volume of stock solution of aspirin= 100ml The dilution factor for the stock solution= 50/2 Molar mass of SAS = 180.157 g/mole c) Measurements of Absorbance: Solution Absorbance SA1 0.154 SA2 0.524 SA3 0.789 SA4 0.989 The unknown sample 0.259
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 86QAP: A martini, weighing about 5.0 oz (142 g), contains 30.0% by mass of alcohol. About 15% of the...
Related questions
Question
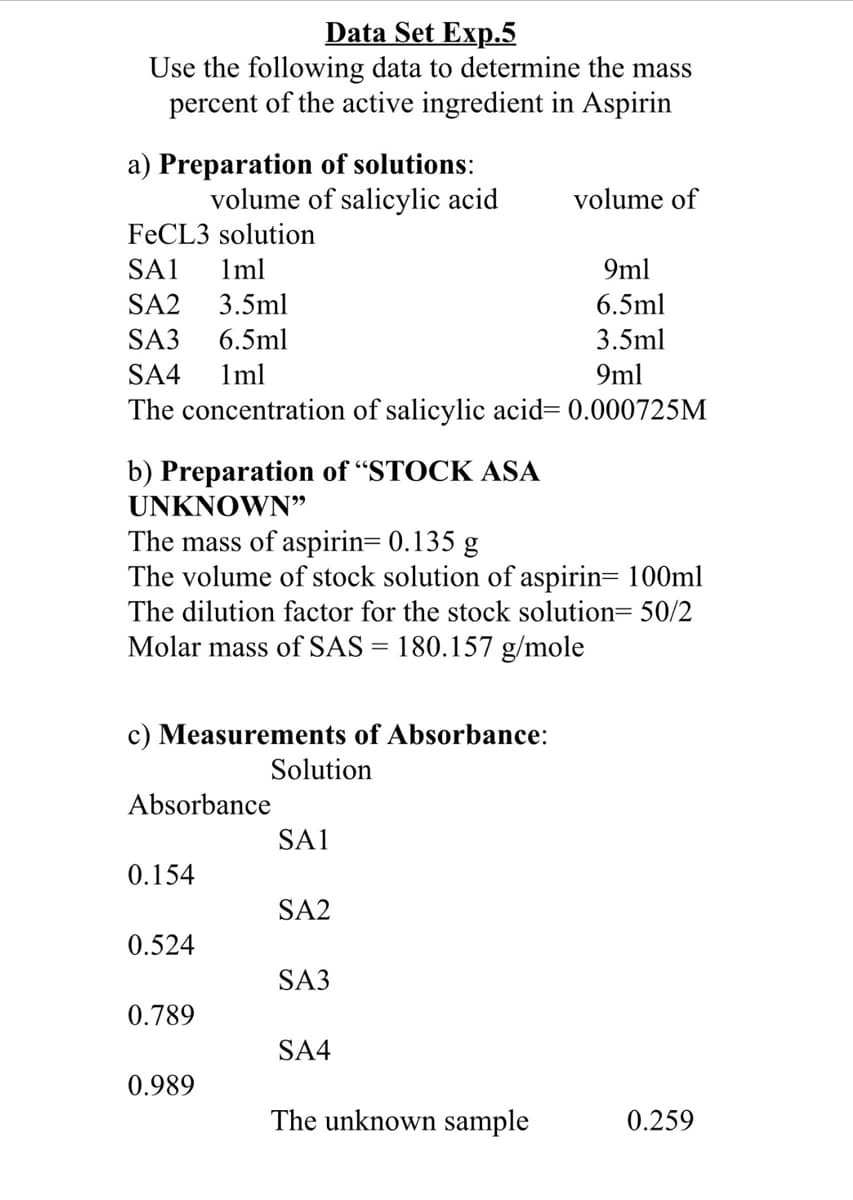
Transcribed Image Text:Data Set Exp.5
Use the following data to determine the mass
percent of the active ingredient in Aspirin
a) Preparation of solutions:
volume of salicylic acid
volume of
FECL3 solution
SA1
1ml
9ml
SA2
3.5ml
6.5ml
SA3
6.5ml
3.5ml
SA4
1ml
9ml
The concentration of salicylic acid= 0.000725M
b) Preparation of "STOCK ASA
UNKNOWN"
The mass of aspirin= 0.135 g
The volume of stock solution of aspirin= 100ml
The dilution factor for the stock solution= 50/2
Molar mass of SAS = 180.157 g/mole
c) Measurements of Absorbance:
Solution
Absorbance
SA1
0.154
SA2
0.524
SA3
0.789
SA4
0.989
The unknown sample
0.259

Transcribed Image Text:A) Standard solution and Beer's law calibration curve
1-Fill in the following table :
Concentration For S.1 M¸ x V = M, x V 0.000725 x 1.0ml = M, x 10.0ml
Solution
Absorbance
Concentration
S. 1
S.2
S.3
S.4
ASA of unknown
666666666
2- Plot a graph of Absorbance vs. conc mol / L
absorbance
Absorbance vs. Concentration
1.6
1.4
1.2
1
0.8
OY-Values
0.6
0.4
0.2
6.
10
conc 104 mol/ L
B) Analvsis of unknown aspirin tablet
1. Mass of aspirin tablet =
g
2. Concentration of ASA unknown from calibration curve
mol / L
3.. Conc of Stock ASA unknown = 50 mL / 1.5 mL (D.F) x Conc of ASA unknown
mol / L
4. Moles of ASA in 100 mL solution = Conc of Stock ASA unknown x 0.1 L=
moles
5.Mass of ASA = moles ASA x Molar Mass ASA (180 g/ mol) =
6. % of ASA in aspirin
Mass of ASA / Mass of aspirin tablet x100 =
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

