A 88.0 ml. solution of 0.100 M of AGNO, reacts with 180.0 ml. solution of 0.201 M of NaCI to produce silver chloride (AgCI) and sodium nitrate (NANO,) according to the balanced equation AGNO, (aq)+NaCI(aq) → AGCI(s) + NANO, (aq) Determine the limiting reactant for the given reaction. ABCI AGNO, NaCI NANO, Calculate the mass of AgCl produced in the given reaction. mass of AgCl: If 0.790 g of AgCI are isolated after carrying out the given reaction, calculate the percent yield of AgCl.
A 88.0 ml. solution of 0.100 M of AGNO, reacts with 180.0 ml. solution of 0.201 M of NaCI to produce silver chloride (AgCI) and sodium nitrate (NANO,) according to the balanced equation AGNO, (aq)+NaCI(aq) → AGCI(s) + NANO, (aq) Determine the limiting reactant for the given reaction. ABCI AGNO, NaCI NANO, Calculate the mass of AgCl produced in the given reaction. mass of AgCl: If 0.790 g of AgCI are isolated after carrying out the given reaction, calculate the percent yield of AgCl.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem ISP: Aqueous solutions of ammonium sulfide and mercury(II) nitrate react and a precipitate forms. (a)...
Related questions
Question
Kindly refer to the attached picture.
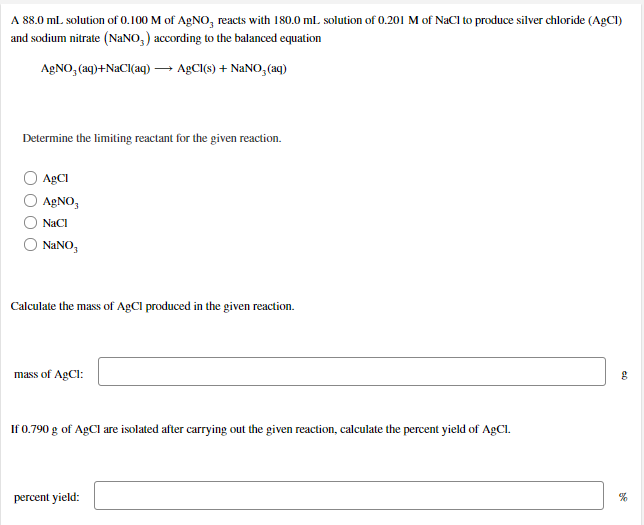
Transcribed Image Text:A 88.0 ml. solution of 0.100 M of AGNO, reacts with 180.0 ml. solution of 0.201 M of NaCI to produce silver chloride (AgCI)
and sodium nitrate (NANO,) according to the balanced equation
AGNO, (aq)+NaCI(aq) → AGCI(s) + NANO, (aq)
Determine the limiting reactant for the given reaction.
ABCI
AGNO,
NaCI
NANO,
Calculate the mass of AgCl produced in the given reaction.
mass of AgCl:
If 0.790 g of AgCI are isolated after carrying out the given reaction, calculate the percent yield of AgCl.
percent yield:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning