a) A basement refrigerator is used to store cool beverages during the long hot summer. The refrigerator is maintained at 2°C and the ambient basement temperature is 20°C. Glass bottles filled with cola at 25°C are placed in the refrigerator. Each bottle weighs 0.20 kg and there is 0.25 kg of cola in each bottle. Assume the glass bottles are in thermal equilibrium with the cola at all times (i.e. the temperature of the bottles and cola are equal). The available energy (work) to cool the bottles of cola in the refrigerator space is 110 kJ. The coefficient of performance of the refrigerator is 0.20 % of that of a Carnot refrigerator. Assume the bottle has the properties of plate glass, and the cola has the properties of saturated liquid water. How many bottles containing cola can be placed in the refrigerator and cooled to 2°C? b) Define the reversible (Q /Q)rev ratio in terms of T, and T. Prove that the coefficient of performance of a reversible heat pump (). is IH TH-TL rev
a) A basement refrigerator is used to store cool beverages during the long hot summer. The refrigerator is maintained at 2°C and the ambient basement temperature is 20°C. Glass bottles filled with cola at 25°C are placed in the refrigerator. Each bottle weighs 0.20 kg and there is 0.25 kg of cola in each bottle. Assume the glass bottles are in thermal equilibrium with the cola at all times (i.e. the temperature of the bottles and cola are equal). The available energy (work) to cool the bottles of cola in the refrigerator space is 110 kJ. The coefficient of performance of the refrigerator is 0.20 % of that of a Carnot refrigerator. Assume the bottle has the properties of plate glass, and the cola has the properties of saturated liquid water. How many bottles containing cola can be placed in the refrigerator and cooled to 2°C? b) Define the reversible (Q /Q)rev ratio in terms of T, and T. Prove that the coefficient of performance of a reversible heat pump (). is IH TH-TL rev
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter3: Transient Heat Conduction
Section: Chapter Questions
Problem 3.12P
Related questions
Question
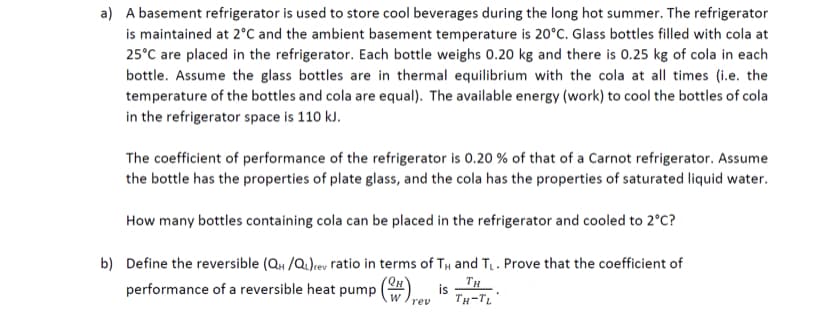
Transcribed Image Text:a) A basement refrigerator is used to store cool beverages during the long hot summer. The refrigerator
is maintained at 2°C and the ambient basement temperature is 20°C. Glass bottles filled with cola at
25°C are placed in the refrigerator. Each bottle weighs 0.20 kg and there is 0.25 kg of cola in each
bottle. Assume the glass bottles are in thermal equilibrium with the cola at all times (i.e. the
temperature of the bottles and cola are equal). The available energy (work) to cool the bottles of cola
in the refrigerator space is 110 kJ.
The coefficient of performance of the refrigerator is 0.20 % of that of a Carnot refrigerator. Assume
the bottle has the properties of plate glass, and the cola has the properties of saturated liquid water.
How many bottles containing cola can be placed in the refrigerator and cooled to 2°C?
b) Define the reversible (Q /Q.)rev ratio in terms of TH and T. Prove that the coefficient of
performance of a reversible heat pump ()
TH
TH-TL"
rev
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning