Chapter44: Preparation Of Methyl Orange
Section: Chapter Questions
Problem 3Q
Related questions
Question
The photographs below (a) show what occurs when a solution of iron(III) nitrate is
treated with a few drops of aqueous potassium thiocyanate. The nearly colorless
iron(III) ion is converted to a red [Fe(H2O)5SCN)
2+
ion. (This is a classic test for the
presence of iron(III) ions in solution.)
[Fe(H2O)6
]
3+
(aq) + SCN
−
(aq) ⇄ [Fe(H2O)5SCN]
2+
(aq) + H2O(ℓ)
(a) As more KSCN is added to the solution, the color becomes even more red.
Explain this observation.
(b) Silver ions form a white precipitate with SCN
−
ions. What would you observe
on adding a few drops of aqueous silver nitrate to a red solution of [Fe(H2O)5 Explain your observations?
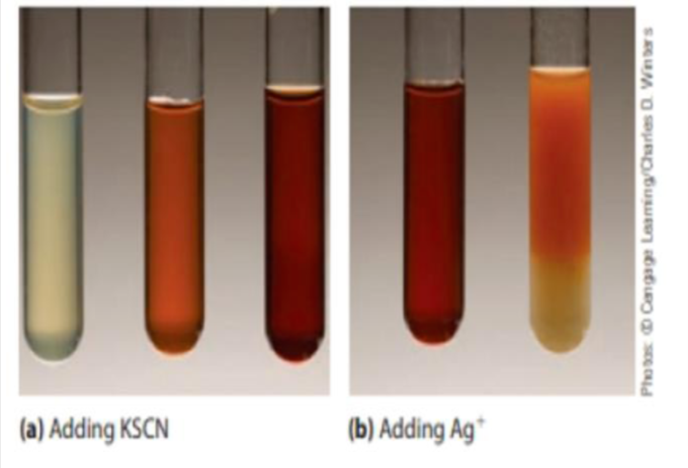
Transcribed Image Text:(a) Adding KSCN
(b) Adding Ag*
Photas D Cangage Laaming/Carles O. Wners
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT