A B 2 3 n=4 Hydrogen Atom Use the Bohr model of the hydrogen atom to determine the properties of the photon that will be emitted by each of the above transitions (by completing the table below). Transition Photon Energy (J) Photon Frequency (Hz) Photon Wavelength (nm) A 1 B C
A B 2 3 n=4 Hydrogen Atom Use the Bohr model of the hydrogen atom to determine the properties of the photon that will be emitted by each of the above transitions (by completing the table below). Transition Photon Energy (J) Photon Frequency (Hz) Photon Wavelength (nm) A 1 B C
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter7: Quantum Theory Of The Atom
Section: Chapter Questions
Problem 7.28QP: An atom in its ground state absorbs a photon (photon 1), then quickly emits another photon (photon...
Related questions
Question
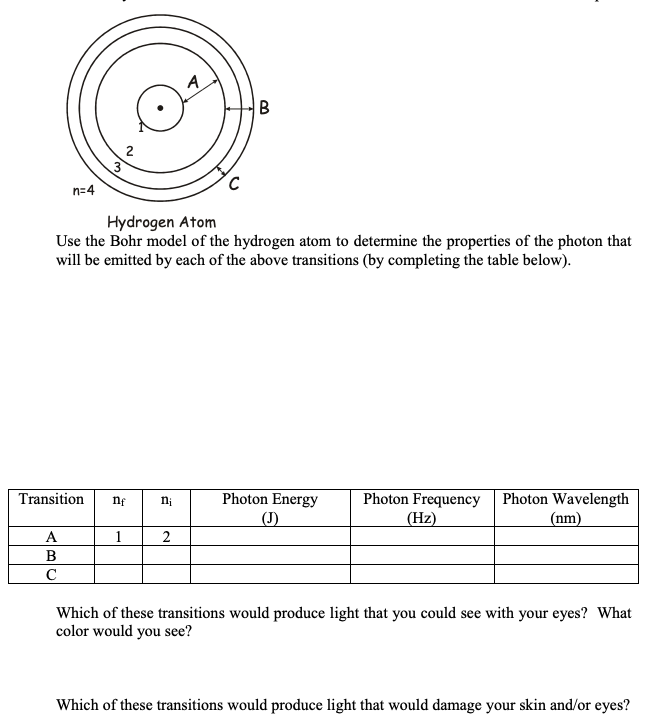
Transcribed Image Text:A
B
2
3
n=4
Hydrogen Atom
Use the Bohr model of the hydrogen atom to determine the properties of the photon that
will be emitted by each of the above transitions (by completing the table below).
Transition
Photon Energy
(J)
Photon Frequency
(Hz)
Photon Wavelength
(nm)
A
1
2
C
Which of these transitions would produce light that you could see with your eyes? What
color would you see?
Which of these transitions would produce light that would damage your skin and/or eyes?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
