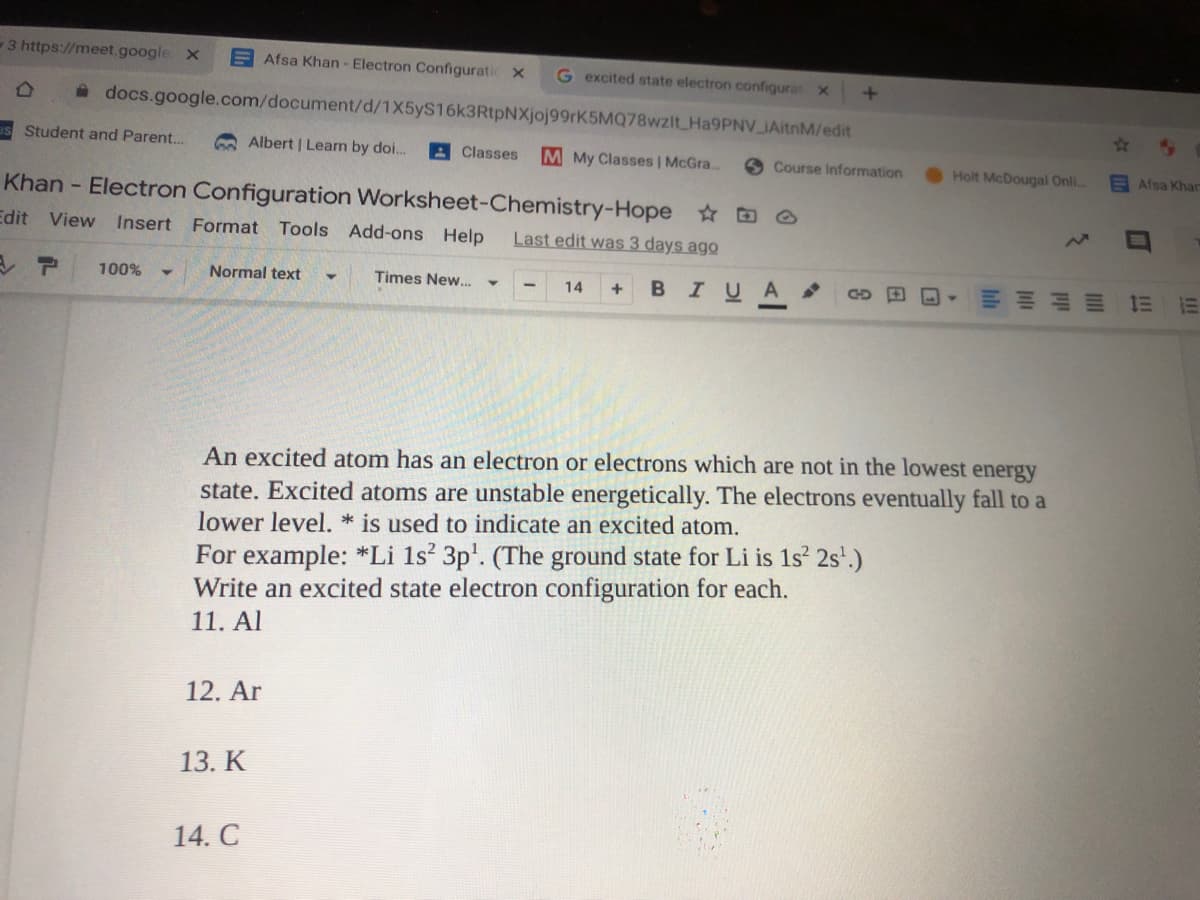
An excited atom has an electron or electrons which are not in the lowest energy state. Excited atoms are unstable energetically. The electrons eventually fall to a lower level. * is used to indicate an excited atom. For example: *Li 1s' 3p'. (The ground state for Li is 1s 2s'.) Write an excited state electron configuration for each. 11. Al 12. Ar 13. K 14. C
An excited atom has an electron or electrons which are not in the lowest energy state. Excited atoms are unstable energetically. The electrons eventually fall to a lower level. * is used to indicate an excited atom. For example: *Li 1s' 3p'. (The ground state for Li is 1s 2s'.) Write an excited state electron configuration for each. 11. Al 12. Ar 13. K 14. C
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 39P: Chapter 3 introduced the concept of a double bond between carbon atoms, represented by C=C , with a...
Related questions
Question
100%
Transcribed Image Text:3 https://meet.google X
E Afsa Khan - Electron Configuratic x
G excited state electron configurat x
à docs.google.com/document/d/1X5yS16k3RtpNXjoj99rK5MQ78wzitHa9PNV IAitnM/edit
us Student and Parent..
A Albert | Leam by doi.
A Classes
M My Classes | McGra...
Course Information
Holt McDougal Onli
EAfsa Khar
Khan - Electron Configuration Worksheet-Chemistry-Hope O
Edit View Insert Format Tools Add-ons Help
Last edit was 3 days ago
100%
Normal text
Times New.
14
IUA
An excited atom has an electron or electrons which are not in the lowest energy
state. Excited atoms are unstable energetically. The electrons eventually fall to a
lower level. * is used to indicate an excited atom.
For example: *Li 1s 3p'. (The ground state for Li is 1s 2s'.)
Write an excited state electron configuration for each.
11. Al
12. Ar
13. K
14. C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning