A battery harnesses a chemical reaction to extract energy in the form of useful electrical work. (a) A certain battery runs a toy truck and becomes par- tially discharged. In the process, it performs a total of 117.0 J of work on its immediate surroundings. It also gives off 3.0 J of heat, which the surroundings absorb. No other work or heat is exchanged with the sur- roundings. Compute q, w, and AU of the battery, making sure each quantity has the proper sign. (b) The same battery is now recharged exactly to its origi- nal condition. This requires 210.0 J of electrical work from an outside generator. Determine q for the battery in this process. Explain why q has the sign that it does.
A battery harnesses a chemical reaction to extract energy in the form of useful electrical work. (a) A certain battery runs a toy truck and becomes par- tially discharged. In the process, it performs a total of 117.0 J of work on its immediate surroundings. It also gives off 3.0 J of heat, which the surroundings absorb. No other work or heat is exchanged with the sur- roundings. Compute q, w, and AU of the battery, making sure each quantity has the proper sign. (b) The same battery is now recharged exactly to its origi- nal condition. This requires 210.0 J of electrical work from an outside generator. Determine q for the battery in this process. Explain why q has the sign that it does.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 10P
Related questions
Question
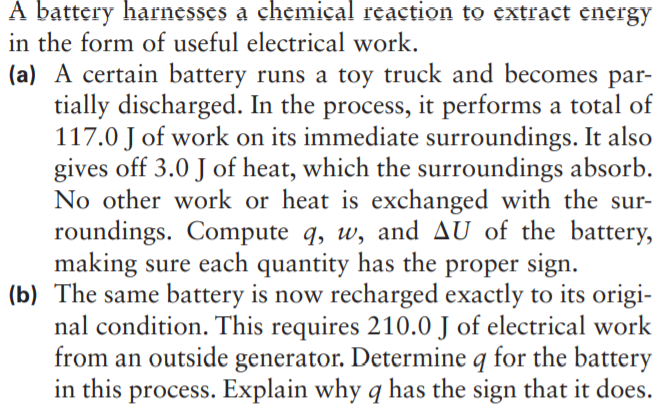
Transcribed Image Text:A battery harnesses a chemical reaction to extract energy
in the form of useful electrical work.
(a) A certain battery runs a toy truck and becomes par-
tially discharged. In the process, it performs a total of
117.0 J of work on its immediate surroundings. It also
gives off 3.0 J of heat, which the surroundings absorb.
No other work or heat is exchanged with the sur-
roundings. Compute q, w, and AU of the battery,
making sure each quantity has the proper sign.
(b) The same battery is now recharged exactly to its origi-
nal condition. This requires 210.0 J of electrical work
from an outside generator. Determine q for the battery
in this process. Explain why q has the sign that it does.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning