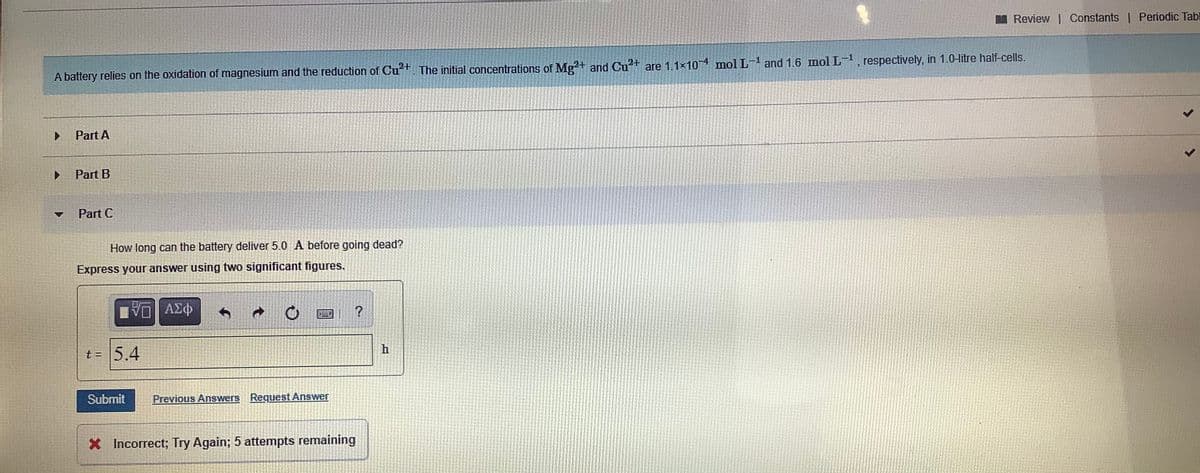
A battery relies on the oxidation of magnesium and the reduction of Cu*+. The initial concentrations of Mg?+ and Cu+ are 1.1x104 mol L-1 and 1.6 mol L- , respectively, in 1.0-litre half-cells. Part A 4. Part B Part C How long can the battery deliver 5.0 A before going dead? Express your answer using two significant figures. t = 5.4 Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining
A battery relies on the oxidation of magnesium and the reduction of Cu*+. The initial concentrations of Mg?+ and Cu+ are 1.1x104 mol L-1 and 1.6 mol L- , respectively, in 1.0-litre half-cells. Part A 4. Part B Part C How long can the battery deliver 5.0 A before going dead? Express your answer using two significant figures. t = 5.4 Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.106QE
Related questions
Question
100%
Can someone please help with question 10. 10.7 and 5.4 are not right answers.
Transcribed Image Text:Review | Constants | Periodic Tabl
A battery relies on the oxidation of magnesium and the reduction of Cu+. The initial concentrations of Mg2+ and Cu?+ are 1.1×10-4 mol L- and 1.6 mol L-, respectively, in 1.0-litre half-cells.
Part A
Part B
Part C
How long can the battery deliver 5.0 A before going dead?
Express your answer using two significant figures.
ΑΣφ
h
t = 5.4
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning




Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning