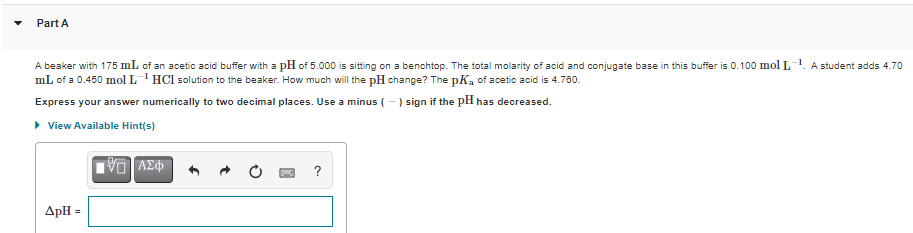
A beaker with 175 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The total molarity of acid and conjugate base in this buffer is 0.100 mol L. A student adds 4.70 mL of a 0.450 mol L HCl solution to the beaker. How much will the pH change? The pKa of acetic acid is 4.760. Express your answer numerically to two decimal places. Use a minus (- ) sign if the pH has decreased. > View Available Hint(s) ? ApH =
A beaker with 175 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The total molarity of acid and conjugate base in this buffer is 0.100 mol L. A student adds 4.70 mL of a 0.450 mol L HCl solution to the beaker. How much will the pH change? The pKa of acetic acid is 4.760. Express your answer numerically to two decimal places. Use a minus (- ) sign if the pH has decreased. > View Available Hint(s) ? ApH =
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 4RQ: A good buffer generally contains relatively equal concentrations of weak acid and conjugate base. If...
Related questions
Question
Transcribed Image Text:Part A
A beaker with 175 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The total molarity of acid and conjugate base in this buffer is 0.100 mol L
mL of a 0.450 mol L'HCl solution to the beaker. How much will the pH change? The pK, of acetic acid is 4.780.
A student adds 4.70
Express your answer numerically to two decimal places. Use a minus (-) sign if the pH has decreased.
• View Available Hint(s)
VO AZ
?
ApH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning