A binary feed is 35.% isopropyl ether (IPE) and the rest isopropyl alcohol (IPA), enters at the bubble point, and needs to be separated into an overhead product containing 65% IPE and a bottom product containing 10.% IPE. A distillation column is to be designed to complete the separation with a total condenser and a partial reboiler. The equilibrium data for an IPA/IPE system at 101 kPa is shown in the figure on the next page. a) Determine the total minimum number of stages required b) Determine the minimum reflux ratio c) Using a reflux ratio equal to 3.5 times the minimum reflux ratio, determine the number of theoretical stages required for the separation using an Rmin of 0.30 (regardless of what you calculated in part b) and the operating conditions listed in the problem statement. *Show lines on graph* d) If the efficiency is 60%, how many actual stages would you need and how many trays? e) Label on the figure which stage is the optimal location of the feed and whether it should be fed above or below the stage. f) Could you obtain an overhead product of 98% ether at 101 kPa starting with this feed? (Yes or No) g) If yes, what needs to happen to get 98%. If no, why not? h) Which is the overall more volatile component? (Ether (IPE) or Alcohol (IPA)) 1.0 0.9 0.8 0.7 0.6 0.5 0.4 E 0.3 0.2 0.1 0.2 0.4 0.6 0.8 1.0 Mole fraction IPE in liquid phase, x 1.0 0.9 0.8 0.7 0.6 0.5 0.4 E 0.3 0.2 0.1 0.2 0.4 0.6 0.8 1.0 Mole fraction IPE in liquid phase, x Mole fraction IPE in vapor phase, y Mole fraction IPE in vapor phase, y
A binary feed is 35.% isopropyl ether (IPE) and the rest isopropyl alcohol (IPA), enters at the bubble point, and needs to be separated into an overhead product containing 65% IPE and a bottom product containing 10.% IPE. A distillation column is to be designed to complete the separation with a total condenser and a partial reboiler. The equilibrium data for an IPA/IPE system at 101 kPa is shown in the figure on the next page. a) Determine the total minimum number of stages required b) Determine the minimum reflux ratio c) Using a reflux ratio equal to 3.5 times the minimum reflux ratio, determine the number of theoretical stages required for the separation using an Rmin of 0.30 (regardless of what you calculated in part b) and the operating conditions listed in the problem statement. *Show lines on graph* d) If the efficiency is 60%, how many actual stages would you need and how many trays? e) Label on the figure which stage is the optimal location of the feed and whether it should be fed above or below the stage. f) Could you obtain an overhead product of 98% ether at 101 kPa starting with this feed? (Yes or No) g) If yes, what needs to happen to get 98%. If no, why not? h) Which is the overall more volatile component? (Ether (IPE) or Alcohol (IPA)) 1.0 0.9 0.8 0.7 0.6 0.5 0.4 E 0.3 0.2 0.1 0.2 0.4 0.6 0.8 1.0 Mole fraction IPE in liquid phase, x 1.0 0.9 0.8 0.7 0.6 0.5 0.4 E 0.3 0.2 0.1 0.2 0.4 0.6 0.8 1.0 Mole fraction IPE in liquid phase, x Mole fraction IPE in vapor phase, y Mole fraction IPE in vapor phase, y
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question

Transcribed Image Text:A binary feed is 35.% isopropyl ether (IPE) and the rest isopropyl alcohol (IPA), enters at the
bubble point, and needs to be separated into an overhead product containing 65% IPE and a
bottom product containing 10.% IPE. A distillation column is to be designed to complete the
separation with a total condenser and a partial reboiler. The equilibrium data for an IPA/IPE
system at 101 kPa is shown in the figure on the next page.
a) Determine the total minimum number of stages required
b) Determine the minimum reflux ratio
c) Using a reflux ratio equal to 3.5 times the minimum reflux ratio, determine the number of
theoretical stages required for the separation using an Rmin of 0.30 (regardless of what you
calculated in part b) and the operating conditions listed in the problem statement. *Show lines
on graph*
d) If the efficiency is 60%, how many actual stages would you need and how many trays?
e) Label on the figure which stage is the optimal location of the feed and whether it should be
fed above or below the stage.
f) Could you obtain an overhead product of 98% ether at 101 kPa starting with this feed? (Yes
or No)
g) If yes, what needs to happen to get 98%. If no, why not?
h) Which is the overall more volatile component? (Ether (IPE) or Alcohol (IPA))
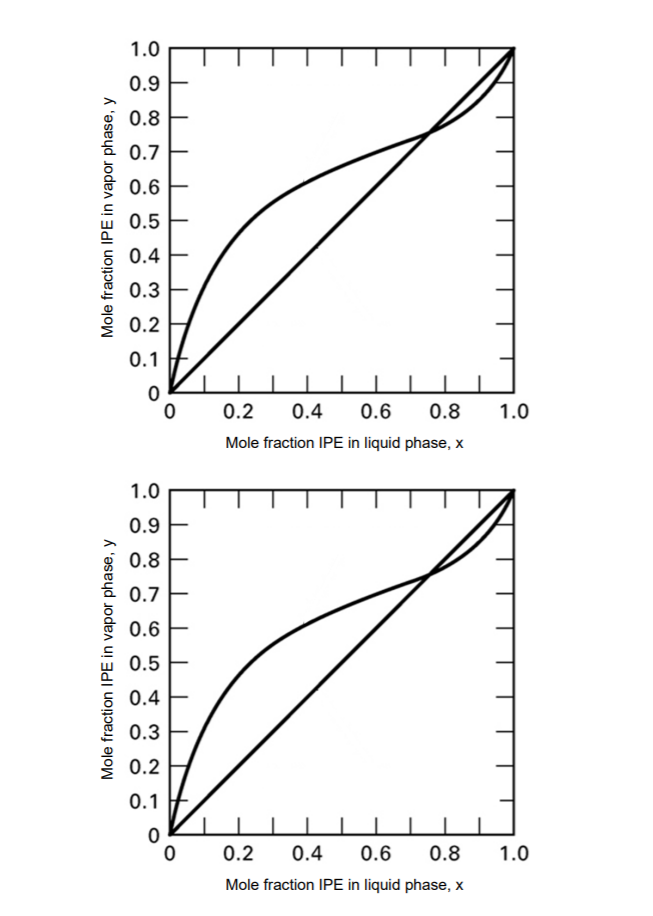
Transcribed Image Text:1.0
0.9
0.8
0.7
0.6
0.5
0.4 E
0.3
0.2
0.1
0.2
0.4
0.6
0.8
1.0
Mole fraction IPE in liquid phase, x
1.0
0.9
0.8
0.7
0.6
0.5
0.4 E
0.3
0.2
0.1
0.2
0.4
0.6
0.8
1.0
Mole fraction IPE in liquid phase, x
Mole fraction IPE in vapor phase, y
Mole fraction IPE in vapor phase, y
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 8 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The