
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 46PS
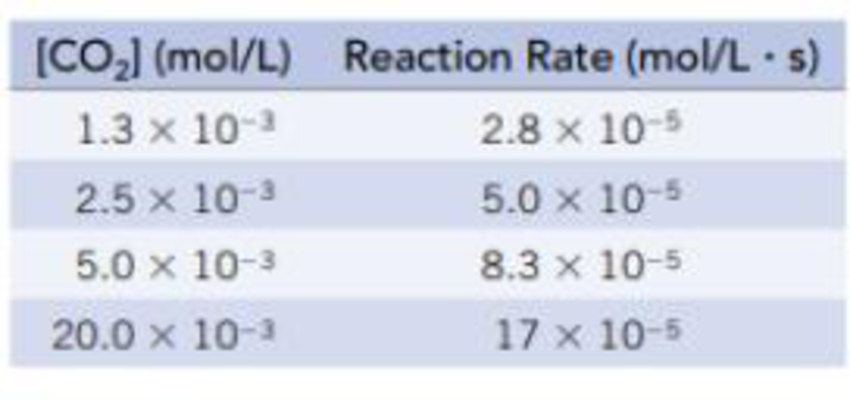
The enzyme carbonic anhydrase catalyzes the transformation of carbon dioxide into hydrogen carbonate ions. This reaction was studied by H. DeVoe and G. B. Kistiakowsky (Journal of the American Chemical Society, Vol. 83, p. 274, 1961) and found to obey the Michaelis-Menten model. Use the data below at a given temperature to calculate the maximum
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Chemistry & Chemical Reactivity
Ch. 14.1 - Sucrose decomposes to fructose and glucose in acid...Ch. 14.1 - What are the relative rates of appearance or...Ch. 14.3 - The initial rate ( [NO]/ t] of the reaction of...Ch. 14.3 - The rate constant, k, at 25 C is 0.27/h for the...Ch. 14.4 - Sucrose, a sugar, decomposes in acid solution to...Ch. 14.4 - Gaseous azomethane (CH3N2CH3) decomposes to ethane...Ch. 14.4 - Prob. 14.7CYUCh. 14.4 - The catalyzed decomposition of hydrogen peroxide...Ch. 14.4 - Americium is used in smoke detectors and in...Ch. 14.5 - Prob. 14.10CYU
Ch. 14.5 - The colorless gas N2O4, decomposes to the brown...Ch. 14.7 - Nitrogen monoxide is reduced by hydrogen to give...Ch. 14.7 - Prob. 14.13CYUCh. 14.7 - One possible mechanism for the decomposition of...Ch. 14.7 - Prob. 1.1ACPCh. 14.7 - Prob. 1.2ACPCh. 14.7 - Prob. 2.1ACPCh. 14.7 - Prob. 2.2ACPCh. 14.7 - Prob. 2.3ACPCh. 14.7 - Determine the activation energy for the reaction...Ch. 14 - Give the relative rates of disappearance of...Ch. 14 - Give the relative rates of disappearance of...Ch. 14 - In the reaction 2 O3(g) 3 O2(g), the rate of...Ch. 14 - In the synthesis of ammonia, if [H2]/t = 4.5 104...Ch. 14 - Experimental data are listed here for the reaction...Ch. 14 - Phenyl acetate, an ester, reacts with water...Ch. 14 - Using the rate equation Rate = k[A]2[B], define...Ch. 14 - A reaction has the experimental rate equation Rate...Ch. 14 - The reaction between ozone and nitrogen dioxide at...Ch. 14 - Nitrosyl bromide, NOBr, is formed from NO and Br2:...Ch. 14 - The data in the table are for the reaction of NO...Ch. 14 - The reaction 2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g)...Ch. 14 - Data for the reaction NO(g) + O2(g) NO2(g) are...Ch. 14 - Data for the following reaction are given in the...Ch. 14 - The rate equation for the hydrolysis of sucrose to...Ch. 14 - The decomposition of N2O5 in CCl4 is a first-order...Ch. 14 - The decomposition of SO2Cl2 is a first-order...Ch. 14 - The conversion of cyclopropane to propene (Example...Ch. 14 - Hydrogen peroxide, H2O2(aq), decomposes to H2O()...Ch. 14 - The decomposition of nitrogen dioxide at a high...Ch. 14 - At 573 K, gaseous NO2(g) decomposes, forming NO(g)...Ch. 14 - The dimerization of butadiene, C4H6, to form...Ch. 14 - The decomposition of ammonia on a metal surface to...Ch. 14 - Hydrogen iodide decomposes when heated, forming...Ch. 14 - The rate equation for the decomposition of N2O5...Ch. 14 - Gaseous azomethane, CH3N=NCH3, decomposes in a...Ch. 14 - The decomposition of SO2Cl2 SO2Cl2(g) SO2(g) +...Ch. 14 - The compound Xe(CF3)2 decomposes in a first-order...Ch. 14 - The radioactive isotope 64Cu is used in the form...Ch. 14 - Radioactive gold-198 is used in the diagnosis of...Ch. 14 - Prob. 31PSCh. 14 - Ammonia decomposes when heated according to the...Ch. 14 - Gaseous NO2 decomposes at 573 K. NO2(g) NO(g) + ...Ch. 14 - The decomposition of HOF occurs at 25 C. HOF(g) ...Ch. 14 - Prob. 35PSCh. 14 - Prob. 36PSCh. 14 - Calculate the activation energy, Ea, for the...Ch. 14 - If the rate constant for a reaction triples when...Ch. 14 - When healed lo a high temperature, cyclobutane,...Ch. 14 - When heated, cyclopropane is converted to propene...Ch. 14 - The reaction of H2 molecules with F atoms H2(g) +...Ch. 14 - Prob. 42PSCh. 14 - Compare the lock-and-key and induced-fit models...Ch. 14 - Prob. 44PSCh. 14 - Prob. 45PSCh. 14 - The enzyme carbonic anhydrase catalyzes the...Ch. 14 - What is the rate law for each of the following...Ch. 14 - What is the rate law for each of the following...Ch. 14 - Ozone, O3, in the Earths upper atmosphere...Ch. 14 - The reaction of NO2(g) and CO(g) is thought to...Ch. 14 - A proposed mechanism for the reaction of NO2 and...Ch. 14 - The mechanism for the reaction of CH3OH and HBr is...Ch. 14 - A reaction has the following experimental rate...Ch. 14 - For a first-order reaction, what fraction of...Ch. 14 - Prob. 55GQCh. 14 - Data for the following reaction are given in the...Ch. 14 - Formic acid decomposes at 550 C according to the...Ch. 14 - Isomerization of CH3NC occurs slowly when CH3NC is...Ch. 14 - When heated, tetrafluoroethylene dimerizes to form...Ch. 14 - Data in the table were collected at 540 K for the...Ch. 14 - Ammonium cyanate, NH4NCO, rearranges in water to...Ch. 14 - Prob. 62GQCh. 14 - At temperatures below 500 K, the reaction between...Ch. 14 - Nitryl fluoride can be made by treating nitrogen...Ch. 14 - The decomposition of dinitrogen pentaoxide N2O5(g)...Ch. 14 - The data in the table give the temperature...Ch. 14 - The decomposition of gaseous dimethyl ether at...Ch. 14 - The decomposition of phosphine, PH3, proceeds...Ch. 14 - The thermal decomposition of diacetylene, C4H2,...Ch. 14 - Prob. 70GQCh. 14 - The ozone in the Earths ozone layer decomposes...Ch. 14 - Hundreds of different reactions occur in the...Ch. 14 - Data for the reaction [Mn(CO)5(CH3CN)]+ + NC5H5 ...Ch. 14 - The gas-phase reaction 2 N2O5(g) 4 NO2(g) + O2(g)...Ch. 14 - Prob. 75GQCh. 14 - The decomposition of SO2Cl2 to SO2 and Cl2 is...Ch. 14 - The decomposition of nitrogen dioxide at a high...Ch. 14 - Prob. 78GQCh. 14 - Egg protein albumin is precipitated when an egg is...Ch. 14 - A The compound 1,3-butadiene (C4H6) forms...Ch. 14 - Hypofluorous acid, HOF, is very unstable,...Ch. 14 - We know that the decomposition of SO2Cl2 is...Ch. 14 - Nitramide, NO2NH2, decomposes slowly in aqueous...Ch. 14 - Prob. 84GQCh. 14 - The color change accompanying the reaction of...Ch. 14 - Prob. 87ILCh. 14 - Prob. 88ILCh. 14 - The oxidation of iodide ion by the hypochlorite...Ch. 14 - The acid-catalyzed iodination of acetone...Ch. 14 - Prob. 91SCQCh. 14 - The following statements relate to the reaction...Ch. 14 - Chlorine atoms contribute to the destruction of...Ch. 14 - Prob. 95SCQCh. 14 - Prob. 96SCQCh. 14 - The reaction cyclopropane propene occurs on a...Ch. 14 - Prob. 98SCQCh. 14 - Examine the reaction coordinate diagram given...Ch. 14 - Draw a reaction coordinate diagram for an...Ch. 14 - Consider the reaction of ozone and nitrogen...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Some bacteria are resistant to the antibiotic penicillin because they produce penicillinase, an enzyme with a molecular weight of 3104 g/mol that converts penicillin into inactive molecules. Although the kinetics of enzyme-catalyzed reactions can be complex, at low concentrations this reaction can be described by a rate equation that is first order in the catalyst (penicillinase) and that also involves the concentration of penicillin. From the following data: 1.0 L of a solution containing 0.15 g ( 0.15106 g) of penicillinase, determine the order of the reaction with respect to penicillin and the value of the rate constant. [Penicillin] (M) Rate (mol/L/min) 2.0106 1.01010 3.0106 1.51010 4.0106 2.01010arrow_forward11.44 A possible reaction for the degradation of the pesticide DDT to a less harmful compound was simulated in the laboratory. The reaction was found to be first order, with k = 4.0 X 10_H s"' at 25°C. What is the half-life for the degradation of DDT in this experiment, in years?arrow_forwardNitrosyl bromide decomposes to nitrogen oxide and bromine. Use the following data to determine the order of the decomposition of nitrosyl bromide.arrow_forward
- For a reaction involving the decomposition of a hypothetical substance Y, these data are obtained: Determine the order of the reaction. Write the rate law for the decomposition of Y. Calculate k for the experiment above.arrow_forwardSucrose decomposes to fructose and glucose in acid solution. A plot of the concentration of sucrose as a function of time is given in the margin. What is the rate of change of the sucrose concentration over the first 2 hours? What is the rate of change over the last 2 hours? Concentration versus time for the decomposition of sucrose.arrow_forwardCreate a table of concentrations, starting with 0.100Mconcentrations of all reactants, that you would proposein order to establish the rate law for the reaction aA+bB+cDproducts using the method ofinitial rates.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY